Quick Look
Grade Level: 4 (3-5)
Time Required: 45 minutes
Expendable Cost/Group: US $2.00
Group Size: 3
Activity Dependency: None
Subject Areas: Physical Science, Science and Technology
NGSS Performance Expectations:

| 4-PS3-2 |
Summary
Students use potatoes to light an LED clock (or light bulb) as they learn how a battery works in a simple circuit and how chemical energy changes to electrical energy. As they learn more about electrical energy, they better understand the concepts of voltage, current and resistance.
Engineering Connection
Engineers use batteries to store energy in a wide range of situations. A solid electrolyte battery is most suitable in very extreme weather conditions, while nickel-zinc batteries work best in electric vehicles. Energy engineers continually evolve technology to improve the performance and life-cycle costs of batteries that store solar and wind energy. When designing a battery, engineers keep in mind the needs of the application, and use different substances to create current flow. They consider characteristics such as power output, ability to recharge, reliability, size, safety, heat generation, length of life cycle, abuse tolerance, cost and ability to be recycled. Isn't the power of engineering creativity amazing?
Learning Objectives
After this activity, students should be able to:
- Describe the flow of electrical energy through a simple circuit.
- Explain why a light bulb may take more than one battery to light up.
- Explain the role of electrical engineers in the development of electricity sources and the products that use electricity.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
4-PS3-2. Make observations to provide evidence that energy can be transferred from place to place by sound, light, heat, and electric currents. (Grade 4) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Make observations to produce data to serve as the basis for evidence for an explanation of a phenomenon or test a design solution. Alignment agreement: | Energy can be moved from place to place by moving objects or through sound, light, or electric currents. Alignment agreement: Energy is present whenever there are moving objects, sound, light, or heat. When objects collide, energy can be transferred from one object to another, thereby changing their motion. In such collisions, some energy is typically also transferred to the surrounding air; as a result, the air gets heated and sound is produced.Alignment agreement: Light also transfers energy from place to place.Alignment agreement: Energy can also be transferred from place to place by electric currents, which can then be used locally to produce motion, sound, heat, or light. The currents may have been produced to begin with by transforming the energy of motion into electrical energy.Alignment agreement: | Energy can be transferred in various ways and between objects. Alignment agreement: |
Common Core State Standards - Math
-
Develop understanding of fractions as numbers.
(Grade
3)
More Details
Do you agree with this alignment?
-
Use the four operations to solve word problems involving distances, intervals of time, liquid volumes, masses of objects, and money, including problems involving simple fractions or decimals, and problems that require expressing measurements given in a larger unit in terms of a smaller unit. Represent measurement quantities using diagrams such as number line diagrams that feature a measurement scale.
(Grade
4)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Energy comes in different forms.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Develop understanding of fractions as numbers.
(Grade
3)
More Details
Do you agree with this alignment?
-
Appropriate measurement tools, units, and systems are used to measure different attributes of objects and time.
(Grade
4)
More Details
Do you agree with this alignment?
Colorado - Science
-
Identify and describe the variety of energy sources
(Grade
4)
More Details
Do you agree with this alignment?
-
Describe the energy transformation that takes place in electrical circuits where light, heat, sound, and magnetic effects are produced
(Grade
4)
More Details
Do you agree with this alignment?
Materials List
To make a potato battery, each group needs:
- 3 copper pennies (or copper strips), one per potato
- 3 zinc nails; these are galvanized nails, available at hardware stores
- 5 insulated wires, 15-20 cm (6-8 inches) long, with alligator clips at the ends
- 1 low-current, light emitting diode (LED) clock (or LCD clock) requiring ~1.5 volts (or a small LED)
- OR above materials can be purchased cumulatively in a kit, available online
- 3 potatoes (fresh)
- Make a Battery Worksheet, one per student
For the entire class to share or one per team:
- (optional) multimeter(s) or voltmeter(s); a multimeter measures current, voltage and resistance of a circuit; available at Radio Shack or other electronics stores or websites
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_energy2_lesson04_activity2] to print or download.Introduction/Motivation
What is electrical energy? From where does it come? Can you think of anything that needs electrical energy to work? How about lamps, music players, TVs and ovens? How do we get the power to run these devices? What role do electrical engineers play in improving our lives?
One place we can find electrical energy in our homes is at a wall outlet. So, how does electrical energy reach the wall outlet in our house? The energy comes from an electrical power plant, which usually makes electrical energy from burning fossil fuels, such as coal and oil. The heat energy from the burning fuel heats up water. When the water boils, it becomes steam, which flows through a pipe into a turbine (a wheel with blades). As the turbine spins from the steam, it turns a generator (a spinning magnet) that unbalances the charges in nearby atoms and produces a current of electricity. The electrical current flows through protected wires to our house.
How else can we power electrical appliances? That's right, from a battery. A battery works by providing electrons with a solution in which they can move around. Have you ever noticed that some batteries have a copper end that is positive (shown with a plus [+] sign) and another end that is negative (shown with a minus [-] sign)? Zinc is a metal that likes to give its electrons to copper. Usually, zinc just gives its electrons to copper and then the process stops. However, if you provide the electrons with a solution (called an electrolyte) to help them move to the copper, and you give the electrons a wire in which they can move from the copper back to the zinc, you can produce a circuit and a flowing path of electrical energy. (Note: Conventional current flows from positive to negative, so from the copper, through the wire, back to the zinc. The electrons actually flow negative to positive.)
A circuit is a complete path of electrical energy. This means that electrical energy or charge is produced or stored somewhere (voltage) and has a path for the charge to flow (or current). Another part of a circuit is a resistance, such as a light bulb. Electrical engineers create circuits to help electrical energy perform work, such as lighting a room or keeping food cold. An appliance, light bulb or almost any device that uses electrical energy is a resistance. A resistance prevents or slows an electrical current or charge from moving. When electrical current flows through a source of resistance, it can be changed into light or heat or sound. Even though you cannot see it, the light bulb or appliance slows down the electrical charge.
Electrical engineers help develop the many modern products and appliances that require electrical energy. Electrical engineers also help create the technology used to generate the electrical energy in power plants in the first place. These engineers know a lot about circuits and they continually work to find better ways to store electrical charge and generate electrical current without using nonrenewable fossil fuels such as coal and oil. These electrical engineers are very important in almost everything we do! Essentially, electrical engineers create improvements for society and help save our planet for future generations. Today, we are going to be electrical engineers and learn more about how electrical energy works in a circuit. We are also going to look at generating electrical current from the energy stored in a potato! Do you think we can light a bulb with a potato? Let's try it!
Procedure
Background
How does a potato battery work? The copper (Cu) atoms attract electrons more than the zinc (Zn) atoms. If you place a strip of copper and a strip of zinc in contact with each other, many electrons pass from the zinc to the copper. As they concentrate on the copper, the electrons repel each other. When the force of repulsion between electrons and the force of attraction of electrons to the copper become equalized, the flow of electrons stops. Unfortunately it is not possible to take advantage of this behavior to produce electricity because the flow of charges stops almost immediately. On the other hand, if you bathe the two strips in a conductive solution, and connect them externally with a wire, the reactions between the electrodes and the solution continually furnish the circuit with charges. In this way, the process that produces the electrical energy continues and becomes useful. In a conductive solution, the charge is carried by ions that move through the solution. In the solid state, the ions are not able to move around freely. However, once they are dissolved in water, they become completely mobile. They can "swim" around in the water and thus can respond to an electric current from a battery. That current supplies electrons that cause positive ions to flow in one direction and negative ions to flow in the opposite direction. The ions carry electrical charge from one electrode to another, completing the electrical circuit.
For a conductive solution, use any electrolyte, whether it is an acid, base or salt solution. An electrolyte is a substance whose aqueous solution contains various ions and thus conducts electricity. Therefore, the more free ions a solution has, the better conductor it will be. Many fruits and vegetables contain juices rich in ions and are therefore good electrical conductors.
Like any battery, a potato battery has a limited life span. The electrodes undergo chemical reactions that block the flow of electricity. The electromotive force diminishes and the battery stops working. Usually, what happens is the production of hydrogen at the copper electrode and the zinc electrode acquires deposits of oxides that act as a barrier between the metal and the electrolyte. This is referred to as the electrodes being polarized. To achieve a longer life and higher voltages and current flows, it is necessary to use electrolytes better suited for the purpose. Commercial batteries, apart from their normal electrolyte, contain chemicals with an affinity for hydrogen, which combine with the hydrogen before it can polarize the electrodes.
Before the Activity
- Gather materials and make copies of the Make a Battery Worksheet.
With the Students
- Divide the class into teams of two or three students each. Hand out the materials.
- Direct groups to carefully place the zinc nails and copper pennies into the potato. Make sure the two different metals do not touch each other in the potato (see Figure 1).
- Connect one alligator clip to the end of the penny sticking out of the potato and another alligator clip to the end of the nail sticking out of the potato (see Figure 1).

- (If you have a multimeter) Tell students that a multimeter is an instrument that measures current, voltage and resistance of a circuit, and is a tool (created by and) often used by engineers. Set the multimeter on a low "DC volts" scale for voltage and "DC milliamps" for current, so students can see the charge that one potato can produce. Expect the potato to produce just less than 1 volt. Encourage students to convert the decimal readout from the multimeter to a fraction (for example, 0.82 volts = 82/100 volts).
- Have the students figure out how many potatoes they need to light their LED clock (or clock). For example, if their potato produces a voltage of 0.8 volts, then they may need two potatoes to power a 1.5 voltage LED.
- Have students experiment to figure out how to connect two potatoes together. To connect two potatoes in series (to add more voltage), place a penny and nail into a second potato, and connect the wire from the zinc nail in the first potato to the copper penny in the second. Then, add a third wire to the zinc nail in the second potato. Always remember to connect the copper (positive) end of the potato (battery) to the zinc (negative) end of the next potato (see Figure 2).
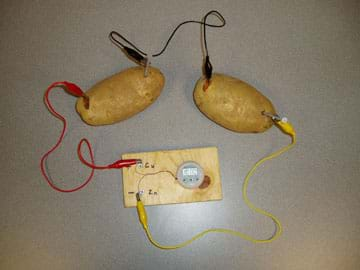
- Expect two potatoes in series to be able to light an LED; however, you might need three. Show students how to connect the LEDs to the potato in the correct manner, that is, the positive end of the LED to the negative end of the potato battery (zinc nail) and the negative end of the LED to the positive end of the potato battery (copper penny).
- Have students discuss how the potatoes provide the electrolyte (solution) for the chemical battery to work. Ask for suggestions of other foods we could try (for example, lemons, berries, apples).
- Ask students to complete the worksheet either individually or in pairs. After they finish, have them compare answers with a peer or another pair, giving all students time to finish.
Vocabulary/Definitions
conductor: An object that allows the transfer of electrons.
current: The movement of electrons.
electrical energy: Energy produced through the movement of electrons (voltage X current).
electrolyte: A solution that conducts electricity.
energy: The ability to do work.
insulator: An object that inhibits the transfer of electrons.
resistance: Objects or substances that prevent the passage of a steady electric current.
voltage: The amount of energy produced.
Assessment
Pre-Activity Assessment
Brainstorming: In small groups, have students engage in open discussion. Remind them that no idea or suggestion is "silly." All ideas should be respectfully heard. Take an uncritical position, encourage wild ideas and discourage criticism of ideas. Ask students how many items they can think of that run on batteries. As a class, record their ideas on the board.
Quick Poll: Before the activity begins, ask the class a question and tally their responses on the board. Ask: Can we get electrical energy from a fruit or vegetable?
Activity Embedded Assessment
Worksheet /Pairs Check: Have students work individually or in pairs on the Make a Battery Worksheet. After they finish, have them compare answers with a peer or another pair, giving all students time to finish the worksheet.
Hypothesize: Ask each group what would happen if we added more potatoes in a row to our circuits? (Answer: Adding more potatoes in a row is the same as adding batteries in a row. The voltage of each piece of potato is added up. Thus, the voltage of our circuit would increase. On the other hand, the current of our circuit would not increase. Current is not dependent on how many potatoes but is related to the size of each potato individually.)
Post-Activity Assessment
Problem Solving: Present the class with the following math problems and ask students to calculate how many of each vegetable or fruit are needed to light the bulb.
- If a lemon produces 1 Volt of energy, how many lemons would you need to light a 2 Volt light bulb? (Answer: 2)
- If a potato produces 0.8 Volts of energy, how many potatoes would you need to light a 1.5 Volt light bulb? (Answer: 2)
- If a potato produces 0.8 Volts of energy, how many potatoes would you need to light a 3 Volt digital clock? (Answer: 4)
- If you have a lemon that produces 1 Volt of energy and an apple that produces 1 Volt of energy, can you light a 3 Volt clock? (Answer: No, you would need one more lemon or apple.)
Diagramming: Engineers must understand how circuits work in order to develop cool new technologies. Draw a picture of your potato battery. Label the battery (voltage) and the resistance (light bulb). Draw an arrow to show the current flow (from the copper end to the zinc end). List two cool products that an engineer could develop that run off a fruit battery.
Troubleshooting Tips
If the LED clock, light or small light bulbs do not work, check the setup of the potato battery. Perhaps the ends are not all connected from negative to positive, or perhaps there is not enough potato voltage. Check the voltage of the potato using a multimeter or voltmeter. Another possibility is having enough voltage, but not enough current to light the bulb, which is why it is recommended to use only very low-volt LED clocks or bulbs. Also, try using more potatoes (i.e., 5 potato halves) to strengthen conductivity. Try different LED colors (for example, the blue LED may work better than the red).
Lemons and oranges also work well for this activity. They work best if you first roll them on a table top, which breaks down the cells inside so more juice flows through the fruit (current).
Some people have more conduction success using copper strips instead of copper pennies (can also wrap the pennies in copper ribbon).
Soaking the potatoes in Gatorade overnight can make them more conductive.
Activity Extensions
At activity end, before students disassemble their potato batteries, it is fun to have the entire class connect their fruit batteries in series, making "a serious tater circle."
Have students try the activity again using different fruits or vegetables! Many fruits and vegetables work, such as lemons, limes, apples and carrots. Have students compare and contrast the performance of different fruits and vegetables.
Have students complete the activity again using an electrolyte solution, such as salt water or vinegar. Have them compare and contrast the performance of different electrolyte solutions.
To add a math component, have students use the multimeter to compare the flow of electricity for several different fruits as well as to the total amount of fruits used. Ask them to graph the results and hypothesize what is happening.
Activity Scaling
Have more advanced students experiment with parallel and series configurations using different numbers of potatoes.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about current electricity and necessary conditions for the existence of an electric current. Students construct a simple electric circuit and a galvanic cell to help them understand voltage, current and resistance.

Students learn that charge movement through a circuit depends on the resistance and arrangement of the circuit components. In one associated hands-on activity, students build and investigate the characteristics of series circuits. In another activity, students design and build flashlights.

Students are introduced to several key concepts of electronic circuits. They learn about some of the physics behind circuits, the key components in a circuit and their pervasiveness in our homes and everyday lives.

Students are introduced to the idea of electrical energy. They learn about the relationships between charge, voltage, current and resistance. In the associated activities, students learn how a circuit works and test materials to see if they conduct electricity.
References
Dictionary.com. Lexico Publishing Group, LLC. Accessed September 28, 2005. (Source of some vocabulary definitions, with some adaptation) http://www.dictionary.com
Phillips, W.D. Chapter 2P: Using a Multimeter. Design Electronics (online textbook) DOCTRONICS Educational Publishing, UK. Accessed September 28, 2005. (Good design, technology and electronics resources.) http://www.doctronics.co.uk/meter.htm
Copyright
© 2005 by Regents of the University of ColoradoContributors
Sharon D. Perez-Suarez; Jeff Lyng; Malinda Schaefer Zarske; Denise W. Carlson; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education, and the National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: July 9, 2020







User Comments & Tips