Quick Look
Grade Level: 6 (5-7)
Time Required: 15 minutes
Lesson Dependency: None
Subject Areas: Chemistry, Physical Science
Summary
Students use the associated activity to learn about atoms and their structure (protons, electrons, neutrons) — the building blocks of matter. They see how scientific discoveries about atoms and molecules influence new technologies developed by engineers.Engineering Connection
Some students may have heard of anti-matter, "phasers" (Star Trek™ laser-like weapons that emit high-energy light bursts to stun or fatally wound an enemy) and "Beam me up, Scotty!" (the Star Trek ™ signal to the transporter room). Imagination is great, and as technology advances and engineers learn more about the building blocks of matter, these types of technologies move from imagination to reality.
Learning Objectives
After this lesson, students should be able to:
- Define a molecule.
- List the basic components and structure of the atom.
- Understand how engineers use their knowledge of atomic structure to design new technologies.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms.
(Grades 6 - 8)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Explain patterns in the number of zeros of the product when multiplying a number by powers of 10, and explain patterns in the placement of the decimal point when a decimal is multiplied or divided by a power of 10. Use whole-number exponents to denote powers of 10.
(Grade
5)
More Details
Do you agree with this alignment?
-
Understand the concept of a ratio and use ratio language to describe a ratio relationship between two quantities.
(Grade
6)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Identify evidence that suggests there is a fundamental building block of matter
(Grade
6)
More Details
Do you agree with this alignment?
Introduction/Motivation
Look around the classroom. What do you think makes up all the items in the classroom? (Possible answers: atoms, matter, solid stuff, etc. Some students may answer more concrete things, such as: desks, walls, air, humans, etc.). All living and non-living things around us are made up of stuff called matter. In fact, any item that has mass and takes up space can be considered matter.
Do you know what the basic building blocks of matter are called? Well, the basic building blocks that make up matter are called atoms. Sometimes two or more atoms bond, or stick together, and form a molecule. A molecule is the smallest part of a substance that still has all the properties of that substance. For example, a water molecule is made up of two hydrogen atoms and one oxygen atom. Sometimes, a molecule is made up of two or more of the same atoms, such as a helium gas molecule. The matter and molecules that make up the world around us are formed mostly by many different atoms bonding together — each having their own properties or attributes.
Atoms are little, but they pack a wallop when their energy is released. Let's start with the basic particles that make up the atom and their associated charges and structure. Atoms consist of three particles: negatively charged electrons, positively charged protons and neutrons, which have a neutral charge. It is easy to remember the types of charges on each of these particles when you use a simple association. For example, protons are positive, and both of those words start with p. Also, neutrons are neutral, which start with n. Then we only have to remember one particle: electrons, and they have a negative charge. Where are all these particles located in an atom? The electrons exist in orbits or shells that spin around the nucleus of the atom, which contains the protons and neutrons. In reality, these shells looks like fuzzy clouds that the electrons move about in.
Engineers use their knowledge of the structure of atoms to do everything from developing new materials (non-stick coatings for frying pans, safer football helmets, carbon fiber for faster cars and lighter prosthetics and bicycles, etc.) to harnessing the energy of nuclear reactions for electricity. They also create machines, such as lasers, to artificially create elements. Lasers are used in the medical and dental fields, as well as in various types of industry. Many of the technologies from the old series Star Trek™ are real possibilities for the future, as scientists and engineers learn more about the makeup of matter. During this lesson, we are going to learn more about matter, and the basic building block of matter — the atom. Let's also think about how we can use our knowledge about matter to understand new engineering technologies.
Lesson Background and Concepts for Teachers
History
The ancient Greeks started the atomic ball rolling. Democritus was the first to theorize that matter was made of small pieces. Leucippus was the first to use the term atom (atomon), which meant "indivisible" in Greek. We now know that the atom is divisible and is made of even smaller pieces — the puzzling subatomic particles. Because the Greeks had no way to test and verify their theories, we had to wait almost 2000 years to confirm that atoms do exist, though not quite the way the Greeks imagined.
In the 16th century, Robert Boyle came up with the notion that there were elements that could not be broken down any further, but it was not until the 18th century that John Dalton reasoned that elements might be made of atoms.
The Atom and Atomic Structure
The basic facts to know about the atom are that it is made up of three basic subatomic particles: 1) electrons (negative charge) that spin in shells around a nucleus that consists of 2) protons (positive charge) and 3) neutrons (neutral charge). Generally, the number of protons and electrons balance out to make the atom have an electrically neutral charge. Electrons that are farthest away from the nucleus of an atom (valence electrons) are the ones that are most easily shared with or transferred to other atoms. The atoms that are missing an electron or share an additional electron are called ions and combine easily with other ions to make molecules.
The number of protons in an atom is called the atomic number. This number determines the element of the atom. Within an element, the number of neutrons may vary, creating the different isotopes or nuclides. For the most part, this does not affect the electrical and chemical behavior of the atom. (There is some exception with the mass of the isotope, as heavier isotopes tend to react more slowly than lighter ones.) There are some things that affect the number of protons and neutrons in the nucleus of an atom, including nuclear fission, nuclear fusion and radioactive decay. Normally, though, the number of electrons is the particle that is most easily changed, because of its lower bonding energy.
Traditionally, the atom was represented as a kind of miniature solar system. Now, scientists understand that if we could see an atom, it would look more like a fuzzy little cloud. In fact, scientists can only predict where an electron might be in its shell using the probability theory: the exact position and momentum of an electron cannot be determined simultaneously.
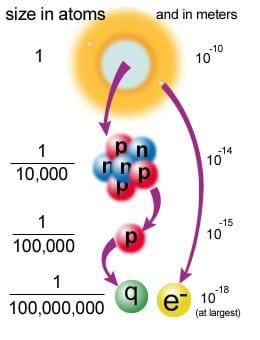
Protons and neutrons are about the same mass; however, electrons are over 1000 times lighter. How small are we talking? Well, as shown in Figure 1, we're talking very, very tiny.
- atom = 1 x 10-10 meters
- nucleus = 1 x 10-15 to 1 x 10-14meters
- neutron or proton = 1 x 10-15 meters
- electron - not known exactly, but thought to be on the order of 1 x 10-18 meters
The atom can be broken down into several, smaller subatomic particles. The three main ones are protons and neutrons, which are found in the nucleus or core of the atom, and electrons, which exist outside of the nucleus. Refer to the Gumdrop Atoms activity to illustrate the anatomy of an atom to give students a better understanding of how these subatomoic particles interact. Physicists have recently divided atoms into even smaller subatomic particles such as fermions (quarks, leptons, neutrinos, electrons) and bosons (gluons, photons, gravitrons). It is difficult (if not impossible) to determine the physical properties of something based on the number or quarks and leptons it contains. The things we see in our world (water, wood, metal, skin, teeth) are better understood and organized by using the number of protons, neutrons and electrons their atoms (and molecules) contain. Similar to how we look at the shapes of different LEGO™ pieces, rather than the plastic that makes them, with this lesson we are just going to look at protons, neutrons and electrons as the as the "LEGOs™" of matter.
Fun Fact: If we drew the atom to scale and made protons and neutrons a centimeter in diameter, then the electrons would be less than the diameter of a hair and the entire atom's diameter would be greater than the length of thirty football fields! In fact, 99.9% of an atom's volume is just empty space! (Source: http://particle.adventure.org/particleadventure/)
Associated Activities
- Gumdrop Atoms - Using gumdrops and toothpicks to make atom models, students learn the basic components of the atom, their charges and basic configuration. They also learn that the atom is made up mostly of space and that electrons move about the nucleus in an electron cloud.
Lesson Closure
So, what is the stuff that is all around us? (Answer: Matter) Matter is anything that has mass and takes up space. The basic building blocks that make up matter are called atoms. What are the different particles found in atoms? (Answer: electrons, protons and neutrons) Where are they found? (Answer: Protons and neutrons are found in the nucleus, and electrons are found in shells around the outside of the nucleus.) Who remembers what a molecule is? (Answer: A molecule is the smallest part of a substance that still has all the properties of that substance; when two or more atoms bond, or stick together, they form a molecule.)
The atom still has many mysteries to discover. In the last 100 years, we have learned new things about how an atom behaves, but there is still so much more to learn. When your parents were growing up, they did not have some of the technology we have today. Advancements made in particle technologies, such as the use of lasers, have occurred because engineers have used the atomic discoveries of scientists to create devices that make our lives better and advance human society. Lasers are used in industry, medicine, military and even many consumer products, such as computers and DVD players.
Vocabulary/Definitions
atom: The basic unit of matter; the smallest unit of an element, having all the characteristics of that element; consists of negatively-charged electrons and a positively-charged center called a nucleus.
atomic theory: The theory that all matter is made up of fundamental particles called atoms; the concept of an atom as being composed of subatomic particles.
electron: Particle orbiting the nucleus of an atom with a negative charge.
molecule: The smallest unit of a substance that retains the chemical and physical properties of the substance; two or more atoms held together by chemical bonds.
neutron: Particle in the nucleus of an atom with no charge.
nucleus: Dense, central core of an atom (made of protons and neutrons).
proton: Particle in the nucleus of an atom with a positive charge.
Assessment
Pre-Lesson Assessment
Discussion Question: Solicit, integrate and summarize student responses.
- Ask students to look around at the items in the classroom, and then ask them what they think the "stuff" is that makes up the items in the classroom. Include technology items, such as computers, telephones and intercoms. (Possible answers: Atoms, matter, solid stuff, etc.)
Post-Introduction Assessment
Voting: Ask a true/false question and have students vote by holding thumbs up for true and thumbs down for false. Count the votes and write the totals on the board. Give the right answer.
- True or False: An atom is the smallest building block of matter (Answer: True)
- True or False: Molecules are made up of two or more atoms. (Answer: True; a molecule is also the smallest part of a substance that still has all the properties of that substance.)
- True or False: Electrons are found in the nucleus of an atom. (Answer: False; electrons are found in shells around the outside of the nucleus.)
- True or False: Engineers use their knowledge of atoms and molecules to develop new technologies. (Answer: True)
- True or False: Lasers are only used in science laboratories. (Answer: False; lasers are used in many things, including industry, dental and medicine, military and consumer products, such as computers and DVD players.)
Lesson Summary Assessment
Flashcards: Each student on a team creates a flashcard with a question on one side and the answer on the other. If the team cannot agree on the answers, they should consult the teacher. Pass the flashcards to the next team. Each member of the team reads a flashcard, and everyone attempts to answer it. If they are right, they can pass on the card to the next team. If they feel they have another correct answer, they should write their answer on the back of the flashcard as an alternative. Once all teams have done all the flashcards, clarify any questions. Sample questions follow:
- Is the charge of a proton positive, negative or neutral? (Answer: Positive)
- What atomic particles exist in the nucleus? (Answer: Protons and neutrons)
Lesson Extension Activities
Be a SME!
People who develop curriculum and training programs frequently rely on a subject matter expert or SME (pronounced "smee") — frequently engineers or other professionals — to give them the latest scoop on the material. For this activity, each student could become a SME on a subject area and give a poster presentation at a "Puzzling Particles" class science fair. Students could individually pick a subatomic particle and become a specialist on that subject. Or, several students could work together to explain atomic structure, for example, demonstrating how electrons move in shells. Students should be encouraged to act out the properties of the particles.
Individually, have students may investigate atoms (via the Internet or other sources).
Students may take the FunBrain Periodic Table interactive quiz at: https://www.funbrain.com/games/periodic-table-game investigate each of the elements via the Internet or other sources.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students use gumdrops and toothpicks to make lithium atom models. Using these models, they investigate the makeup of atoms, including their relative size.

This lesson introduces the concept of electricity by asking students to imagine what their life would be like without electricity. Students learn that electrons can move between atoms, leaving atoms in a charged state.

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students come to understand static electricity by learning about the nature of electric charge, and different methods for charging objects. In a hands-on activity, students induce an electrical charge on various objects, and experiment with electrical repulsion and attraction.
References
Andrew Rader Studios, Rader's Chem4Kids.com, Atom Basics: Overview, "Atoms Around Us," http://www.chem4kids.com/ Accessed August 31, 2006.
Batchelor, David Allen. NASA Goddard Space Flight Center, "The Science of Star Trek," 1993.
Brown, Judy. Miami Museum of Science, Atom's Family, "The Phantom's Portrait Parlor," http://miamisci.org/af/sIn/phantom/index.html.
Particle Data Group of Lawrence Berkeley National Laboratory, "The Particle Adventure: the fundamentals of matter and force," 2002.
Thomas Jefferson National Accelerator Facility - Office of Science Education, Science Education, Games & Puzzles, "It's Elemental: The Periodic Table of Elements," http://education.jlab.org/inexpages/elementgames.html, accessed August 31, 2006.
MacIntyre, Stacy. U.S. Department of Energy, Energy Information Administration, Energy Kid's Page, Energy Facts, "Sources of Energy," http://www.eia.doe.gov/kids/ Accessed August 31, 2006.
University of Colorado at Boulder, Center for Integrated Plasma Studies, Physics 2000, December 2004, http://www.colorado.edu/physics/ Accessed August 31, 2006.
Copyright
© 2006 by Regents of the University of Colorado.Contributors
Brian Kay; Daria Kotys-Schwartz; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: May 28, 2019







User Comments & Tips