Quick Look
Grade Level: 11 (9-12)
Time Required: 45 minutes
Expendable Cost/Group: US $2.00
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Measurement, Physics, Science and Technology
NGSS Performance Expectations:

| HS-PS3-3 |

Summary
Students learn about how a device made with dye from a plant, specifically cherries, blackberries, raspberries and/or black currents, can be used to convert light energy into electrical energy. They do this by building their own organic solar cells and measuring the photovoltaic devices' performance based on power output.Engineering Connection
To address growing energy needs, many engineers are focused on optimizing efficiency in harvesting solar energy. They know that sometimes the most efficient device is not cost effective so a balance between material cost and device performance must be made.
Learning Objectives
After this activity, students should be able to:
- Describe how energy is transferred and converted from sunlight in order to power a device.
- Define organic solar cell.
- List the benefits and drawbacks of organic solar cells.
- Define renewable energy.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS3-3. Design, build, and refine a device that works within given constraints to convert one form of energy into another form of energy. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design, evaluate, and/or refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | At the macroscopic scale, energy manifests itself in multiple ways, such as in motion, sound, light, and thermal energy. Alignment agreement: Although energy cannot be destroyed, it can be converted to less useful forms—for example, to thermal energy in the surrounding environment.Alignment agreement: Criteria and constraints also include satisfying any requirements set by society, such as taking issues of risk mitigation into account, and they should be quantified to the extent possible and stated in such a way that one can tell if a given design meets them.Alignment agreement: | Energy cannot be created or destroyed—it only moves between one place and another place, between objects and/or fields, or between systems. Alignment agreement: Modern civilization depends on major technological systems. Engineers continuously modify these technological systems by applying scientific knowledge and engineering design practices to increase benefits while decreasing costs and risks.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
It is impossible to build an engine to perform work that does not exhaust thermal energy to the surroundings.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Energy resources can be renewable or nonrenewable.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
design, construct, and calculate in terms of current through, potential difference across, resistance of, and power used by electric circuit elements connected in both series and parallel combinations;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 1 indium tin oxide/titanium dioxide-coated plate; possible sources include the Dutch company Man Solar at http://www.mansolar.nl/.
- 1 uncoated glass plate, available in kit from mansolar.com
- 1 pencil
- 1 paper clip
- 2 alligator clips with wire leads
- a light source, such as a microscope lamp
- a device to power, such as a calculator, musical greeting card or small motor
To share with the entire class:
- 10 mL anthocyanin dye from black cherry concentrate, available online at amazon.com, newegg.com, or buy.com
- 1 Petri dish
- 10 mL iodine in potassium iodide electrolyte solution; put in small dropper bottle
- 1 small (<20mL-size) dropper bottle
- ethanol, to rinse glass plates to remove dye
Pre-Req Knowledge
Students should be familiar with electrical circuits.
Introduction/Motivation
The sun is the source of all energy on the Earth. How do we get energy from the sun? (Possible answers: indirectly by eating plants and burning fossil fuels, or directly through solar radiation.) Today we are going to harness some energy from the sun (or a lamp) using organic solar cells.
First of all, what is organic? (Answer: Contains carbon.) So an organic solar cell is different than an inorganic solar cell in that it has carbon-containing compounds doing the work. Inorganic solar cells use compounds such as silicon to do the work. Okay, so what is a solar (or photovoltaic) cell? (Answer: A device that converts sunlight directly into energy.)
Why would we want to get energy from the sun when we can just plug in our TV or pop a battery into our calculator? (Answer: Limited energy resources.) We can use the electricity that comes via the plug, but where does that come from ? Well, it most likely comes from a power plant that burns coal or natural gas to generate electricity. And, from where do coal and natural gas come? (Answer: Ancient plants that have been transformed over millennia into useable fuel; the plants originally received their energy from the sun.) What do we call this type of energy? (Answer: A nonrenewable source of energy.) Fossil fuels are considered nonrenewable because it takes much longer to make coal (or oil or natural gas) than it does for humans to burn it. If we burn all the coal, we cannot just wait a day and then get some more. The same goes for batteries. Once you use a battery, it is used up (it dies), and you have buy more; But, the number of batteries in the world is limited, too. Traditional batteries also use materials, such as nickel, cadmium and lead, which are also limited resources that are not easily recycled. The environmental impact of discharged batteries leads to heavy metal contamination; that's why batteries should never be thrown in the trash.
Because of the disadvantages of using nonrenewable fossil fuels and/or batteries, we want to turn to different sources of energy—sources that are considered renewable. Can you give me some examples? (Answer: Energy from the sun, wind, water, geothermal, etc.) The sun is our greatest renewable energy source, but how can we turn that energy into something we can use?
(Draw a flow of energy on the board as you discuss it.) Energy leaves the sun in the form of light and heat. Some devices collect the heat for energy, but the devices we are going to make today collect the light. The sun releases a wide variety of light along the electromagnetic spectrum. (Show students a diagram of the electromagnetic spectrum, or Figure 1.) The electromagnetic spectrum ranges from low-energy radio waves, such as those you use to listen to music in your car, to high-energy gamma rays, which are emitted from some radioactive elements. What part of the electromagnetic spectrum do you think the sun is emitting? (Answer: The sun emits energy from the UV to the infrared region [250–2500 nm].)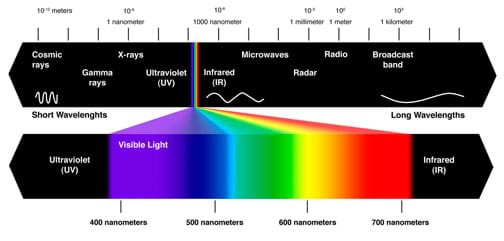
In our device, we have an organic compound called anthocyanin. We extract this compound from plants such as cherries, blackberries and raspberries, by boiling them in water and keeping the purple-red water that contains the dye. Since this compound is colored, it absorbs light. What part of sunlight would the anthocyanin be absorbing? (Answer: Green light; indicate the very small part of the spectrum that is green.) So this molecule only absorbs a tiny part of what the sun emits. In a device, the dye absorbs energy in the form of light and then the light can be turned into electrical charges in the dye, which can flow from the device in the form of electrical energy. But, not just any colored compound will do.
The conversion of light into electrical charges depends upon the chemical makeup of the dye. We chose anthocyanin because it has the right chemical properties for an organic solar cell. Anthocyanin is a small molecule only a few nanometers across. Just for a frame of reference, how thick do you think a single hair is? (Answer: About 10,000 nm thick.) Since it is so small it takes a lot of individual molecules to make a single device. In order to get as much dye as possible in our device, one of the glass slides you will receive (hold up an indium tin oxide/titanium dioxide-coated slide) is coated with indium tin oxide and titanium dioxide. This serves two purposes: the titanium dioxide is nanoporous—like a nanoscale sponge to soak up the dye—and indium tin oxide is the anode where the electrical charge will go into our device. In order to have electrical current you must have a flow of electrons. The electrons will go to the dye from the anode, but where will they go?
We will take another glass slide, one that is not coated with titanium dioxide, and cover it with graphite from a pencil. The graphite acts as the cathode, or where the electrons flow when they come out of the dye. We will also use an electrolyte to ensure good connectivity between all the components. An electrolyte is a substance that has free ions, which makes it a good conductor of electricity. Once you have constructed your device you will test the current it produces by powering different devices.
Procedure
Background
Photovoltaic systems convert light energy into electricity. They are commonly used to power small devices such as calculators and wristwatches. Larger solar cells are used to reduce the energy cost of homes and to power lighted signs. While on average 1,367 watts of solar energy strike a square meter of Earth, we lack the technology to efficiently harvest this light. The most efficient (and most expensive) solar cells approach conversion of ~40 %; these devices are not economically feasible. Maintenance of delicate and expensive solar cells is not competitive with inexpensive and accessible fossil fuels. Other devices using nanocrystalline dyes such as anthocyanins (see Figure 2) have efficiencies of only a few percent, but the cost is so low that it makes optimization of this type of device much more likely to be useful.
When a dye molecule absorbs a photon of light, it forms an excited state that causes an electron to become unpaired and available to be used to create an electrical current. Indium tin oxide is the most commonly used anode in photovoltaic devices because it is transparent. Cathodes are typically metals, but graphite is a much simpler conductor for use in making a device in the classroom. Having an anode and cathode that cause charge flow is necessary to harvest the electrons as they are formed from the light-excited dye molecules.
Before the Activity
- Gather and assemble materials needed for each group.
- Fill a Petri dish with enough anthocyanin to submerge a slide.
- Prepare a small dropper bottle with iodine in potassium iodide electrolyte solution.
- Keep the Petri dish and dropper bottle at the teacher's desk.
With the Students
- Demonstrate each step for students before allowing them to begin (refer to the diagram in Figure 3).

Figure 3. A schematic drawing of an organic solar cell (left) and a finished organic cell device (the activity uses alligator clips instead of solder).
- Divide the class into groups of four students each.
- Have one student from each group prepare the anode by soaking the titanium dioxide-coated plate in the anthocyanin dye for 5 minutes.
- While the anode is being prepared, have another student in each group prepare the cathode by thoroughly covering one side of the uncoated glass slide with graphite from a pencil.
- With the cathode graphite-side up, have each student add 2 drops of electrolyte solution to it (refer to Figure 3).
- Place the anode titanium dioxide down on top of the cathode (refer to Figure 3). Slightly offset the glass plates, and paper clip the glass plates together.
- Connect one alligator clip to the anode and the other to the cathode to complete the circuit with your device. Observe.
- Combine devices with other groups' in a series to power more devices.
- Have students note the temperature of the devices after use.
- Disassemble the devices and rinse the glass plates with ethanol to remove dye.
- Conclude by having students prepare summary lab write-ups, as described in the Assessment section.
Vocabulary/Definitions
anode: An electrode through which electric current flows into a device.
anthocyanin: An organic compound extracted from plants such as cherries, blackberries and raspberries by boiling them in water and keeping the purple-red water that contains the dye that can absorb light energy. Anthocyanins are used in organic solar cells because of their strong light harvesting, and their ability to convert this light energy into electrical energy.
cathode: An electrode through which electric current flows from a device.
electrolyte: A substance with free ions that is electrically conductive.
nonrenewable energy: Energy that cannot be replaced in less time than it takes to be consumed, for example, coal, oil, natural gas
organic: A compound that contains the element carbon.
renewable energy: Energy that automatically replenishes itself from ongoing natural processes, for example, energy from the sun, wind, water, biological, geothermal.
solar cell: A device that converts sunlight directly into electricity.
Assessment
Pre-Activity Assessment
Warm-Up Written Q&A: Have students answer the following questions in written format in their journals:
- What is renewable energy?
- What is an electrical circuit?
- What has more energy, gasoline or sunlight? Why?
Activity Embedded Assessment
Terminology & Content Monitoring: Monitor students' progress throughout the lab by asking them to apply vocabulary to what they are doing. Require that students record their observations in preparation for final lab write-ups. Ask probing questions to get them to refer back to the introduction, for example:
- What is that piece called?
- What is the purpose of (the dye, the glass, the alligator clip, etc.)?
- How does the device work if it is cold outside? (Answer: This is a photovoltaic cell. It converts light to electricity. It does not convert heat to electricity. A device that converts heat to electricity is called a solar thermal cell. A photovoltaic cell works the same on a cold day as a hot day, but it does not work as well on a cloudy day as on a bright day.)
- What happens if (you leave out a piece, you disconnect something, you change the order assembly, etc.)?
Post-Activity Assessment
Lab Write-Ups: Have students report their results, answering the following questions:
- What electrical devices were you able to operate?
- Did your device work?
- What did you observe?
- How did your device compare to others?
In the write-ups, also have students discuss and analyze their results, for example:
- Is this an effective way to power things?
- Why did your device perform well/not perform?
- How could you have improved your device?
Investigating Questions
Why does the device get hot? (Answer: No device is perfect. Not all the light energy taken in by the device comes out of it as electricity. Some of the absorbed light energy is released as heat.)
How could you make your device perform better? (Possible ideas: Having a brighter light source, using more/less dye by soaking it for different amounts of time, using more/less electrolyte solution, using a different dye, heating the dye, coating with graphite more evenly; any parameter can be investigated to find improvements.)
How many of these devices would it take to power your mp3 player/cell phone/computer? (Answer: Respectively, probably more than 500, 3000, and 70,000, respectively.)
Safety Issues
- Iodine and dyes may stain so use caution when handling.
- Glass slides are fragile so handle with care.
Troubleshooting Tips
If a device fails to perform, have students add additional electrolyte. If this fails, reapply graphite and soak in the dye for another five minutes. Make sure to test the light source in advance so you know it is strong enough. Double check that student devices are getting good connectivity between all components.
Activity Scaling
- For lower grades, conduct this activity as a demonstration only.
- For upper grades, provide students with multimeters to measure current and voltage output, and give them more variables to test to optimize device performance.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn how the sun can be used for energy. They learn about passive solar heating, lighting and cooking, and active solar engineering technologies (such as photovoltaic arrays and concentrating mirrors) that generate electricity.
References
Black Current with Blackberry Juice and Sunlight. Products and Prices, Man Solar. Accessed January 3, 2012 (Possible source for initial solar cell kit) http://www.mansolar.nl/
Grätzel, Michael. Dye-sensitized solar cells. Published October 31, 2003. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, Volume 4, Issue 2, pps. 145-153, ISSN 1389-5567, 10.1016/S1389-5567(03)00026-1. Accessed January 10, 2012. http://www.sciencedirect.com/science/article/pii/S1389556703000261.
Man Solar. The Netherlands. Accessed January 10, 2012. (The largest worldwide supplier of turnkey solar cell kits, including educational kits and materials for the preparation of dye-sensitized solar cells, which mimic photosynthesis of green plants. Good information on the website, too.) http://www.mansolar.nl/
Mortimer, Roger J. and David R. Worrall. September 2007 issue. Harnessing Solar Energy with Grätzel Cells. Department of Chemistry, Loughborough University, Loughborough, Leicestershire. Education in Chemistry, Royal Society of Chemistry. Accessed January 10, 2012. (Includes good photos and diagrams of a similar lab.) http://www.rsc.org/Education/EiC/issues/2007Sept/HarnessingSolarEnergyGratzelCells.asp
Nanocrystalline Solar Cell Kit with materials for five reusable cells (order #98-001). Also replacement parts (extra glass slides, kit components and parts). Hands-On Science Kits and Demos, Institute for Chemical Education (ICE), Department of Chemistry, University of Wisconsin-Madison. Accessed January 3, 2012. (Possible source for initial solar cell kit) http://ice.chem.wisc.edu/Catalog/SciKits.html#Anchor-Nanocrystalline-41703
Shallcross, Dudley, Tim Harrison, Steve Henshaw and Linda Sellou. Looking to the Heavens: Climate Change Experiments. August 12, 2009. Science in School, EIROforum, Issue 12. Accessed January 3, 2012. (Source for background information; good photos and diagrams of lab; good information about Grätzel cells and energy from sunlight) http://www.scienceinschool.org/2009/issue12/climate#w3
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 University of HoustonContributors
Crystal YoungSupporting Program
National Science Foundation GK-12 and Research Experience for Teachers (RET) Programs, University of HoustonAcknowledgements
This digital library content was developed by the University of Houston's College of Engineering under National Science Foundation GK-12 grant number DGE 0840889. However, these contents do not necessarily represent the policies of the NSF and you should not assume endorsement by the federal government.
Last modified: March 21, 2023



User Comments & Tips