Quick Look
Grade Level: 6 (6-7)
Time Required: 1 hour
Lesson Dependency: None
Subject Areas: Physical Science, Science and Technology
NGSS Performance Expectations:

| MS-PS3-5 |
Summary
This lesson covers concepts of energy and energy transfer, with a focus on energy transfer in musical instruments. More specifically, students learn the two different ways in which energy can be transferred between a system and its environment. The law of conservation of energy is also described. Example systems are presented (two cars on a track and a tennis ball falling to the ground) and students make predictions and explain the energy transfer mechanisms. The engineering focus becomes clear in the associated activity when students apply the concepts learned in the lesson to design musical instruments. The systems analyzed in the lesson help in discussing how to apply conservation of energy and energy transfer to make things.Engineering Connection
Engineers must understand energy transfer to design instruments that produce beautiful music. Energy transfer is a central concept in the majority of engineering designs.
Learning Objectives
At lesson end, students should be able to:
- Define energy, heat, energy transfer, work and thermal equilibrium.
- Explain how to utilize the concept of energy transfer to make a musical instrument.
- State the law of conservation of energy.
- Define a system and environment in terms of energy, and describe how energy transfer occurs between the two.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS3-5. Construct, use, and present arguments to support the claim that when the kinetic energy of an object changes, energy is transferred to or from the object. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Science knowledge is based upon logical and conceptual connections between evidence and explanations. Alignment agreement: Ask questions that can be investigated within the scope of the classroom, outdoor environment, and museums and other public facilities with available resources and, when appropriate, frame a hypothesis based on observations and scientific principles.Alignment agreement: Apply scientific ideas to construct an explanation for real-world phenomena, examples, or events.Alignment agreement: | When the motion energy of an object changes, there is inevitably some other change in energy at the same time. Alignment agreement: | Energy may take different forms (e.g. energy in fields, thermal energy, energy of motion). Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Energy is the capacity to do work.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
North Carolina - Science
-
Understand characteristics of energy transfer and interactions of matter and energy.
(Grade
6)
More Details
Do you agree with this alignment?
-
Understand forms of energy, energy transfer and transformation and conservation in mechanical systems.
(Grade
7)
More Details
Do you agree with this alignment?
-
Explain how energy can be transformed from one form to another (specifically potential energy and kinetic energy) using a model or diagram of a moving object (roller coaster, pendulum, or cars on ramps as examples).
(Grade
7)
More Details
Do you agree with this alignment?
Introduction/Motivation
Demo: Stretch a rubber band and release it. Then explain to the class that the transfer of energy from potential energy in its stretched state to kinetic energy in the flying rubber band is responsible for the rubber band flying across the room.
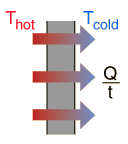
Energy is the driving force of life and is fundamentally responsible for airplanes, cars, and a baseball flying into the stands. What will happen to an extremely hot pot of water if kept in the room at room temperature for a long time? That's right, over time it will cool down, and if enough time passes, we will be able to put our hands in water. The reason the water in the pot cools down over time is due to energy transfer in the form of heat being transferred from the warm object (water) to the cooler object (room) until eventually they reach the same temperature. This is a naturally occurring example of energy transfer.
In the associated activity Energetic Musical Instruments, you will see how musical instruments produce beautiful music using energy transfer.
Lesson Background and Concepts for Teachers
The lesson teaches students concepts of energy and energy transfer. In a simple sense, examples of objects possessing energy are a car traveling down the highway or a raindrop falling from the sky. These are obvious examples of bodies possessing overall kinetic energy (energy of motion). In a microscopic sense, objects also possess internal energy, energy associated with its molecules and atoms. When a set of objects is defined and contained in an environment, this set of objects is defined as a system. For instance, in the aforementioned demonstration, the hot pot of water is the system and the room is the environment. The bodies within the system contain a certain amount of energy initially, whether it be overall kinetic or potential energy or microscopic kinetic or potential energy (internal energy).

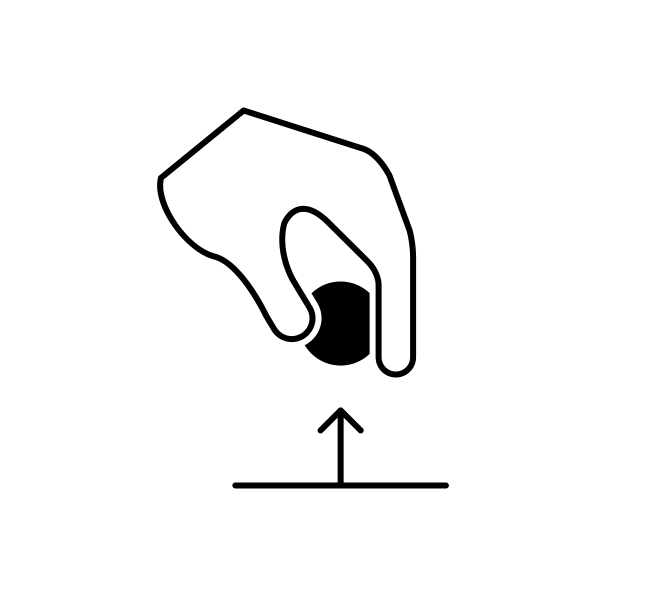
There are two ways that a system can exchange energy with its environment: work done by its environment and heat. In terms of heat, the system originally contains a certain amount of internal energy due to the vibration of its molecules. When the system is originally at a different temperature than its environment, energy will be transferred (heat) between the system and its environment until the system and its environment are at the same temperature, whereby thermal equilibrium is established. The other way energy is exchanged is by external work, or work done by the environment. A simple example of this is a block that is initially resting on the ground. Upon application of a large enough upward force by the hand of a person, the block will be lifted a certain distance. Energy is thus transferred from the person (environment) to the block (system). The environment gave up some energy, and the system accepted the energy.
Associated Activities
- Energetic Musical Instruments - Students apply the principles of energy transfer to design their own musical instruments. This process gives them an introduction to the process of engineering a practical device based upon underlying physical principles.
Lesson Closure
In the macroscopic sense, bodies as part of a system can possess overall kinetic energy (energy of motion) or potential energy. Each system also contains a certain amount of internal energy, energy associated with the kinetic and potential energies of the system's molecules or atoms.
Ask the students to explain the energy transfer mechanisms associated with the rubber band flying across the room at the beginning of the lesson.
Energy can be transferred by either work done by the outside environment or by the transfer of internal energy (heat).
Thermal equilibrium is the condition where two or more objects in thermal contact, meaning they are not insulated from each other, will cease to exchange internal energy.
Vocabulary/Definitions
energy: The capacity or ability to do work
heat: The transfer of energy from one object to another due to temperature differences
internal energy: All the energy of a system that is associated with its microscopic components- atoms and molecules
kinetic energy: The energy an object has because it is moving.
potential energy: The energy an object has because of its position, or condition, rather than motion. A raised weight, coiled spring, or charged battery all have potential energy.
thermal equilibrium: The situation in which two objects in thermal contact cease to exchange energy by the process of heat, i.e. they are at the same temperature
work: The application of a force on a body that transfers energy to that body.
Assessment
- Take a tennis ball (or some other ball) and drop it from the height of the nose. The ball will not bounce as high as it was dropped. Ask the students to explain why. The explanation should go as follows: the ball has a certain amount of energy as it is falling down and upon collision, the ball deforms, which increases the internal energy of the air inside the ball, and by the conservation of energy, this decreases the kinetic energy of the ball causing it not to bounce up to its original height. Also, friction between the ball and the air causes the kinetic energy of the ball to be converted into heat. The force acting against the ball while it is in air is known as air resistance or drag.
- Hit a metal pan with a metal spoon. The students should be able to explain that the kinetic energy of the spoon transfers energy to the metal pan, which begins vibrating. Meanwhile, sound is produced from the collision that carries with it energy. The students should be able to explain that some of the original kinetic energy of the spoon went into vibration of the metal pan and some of it went into sound upon collision. This demonstration can involve hitting any two objects together that will make noise.
- The students should be able to accurately explain why the rubber band is flying across the room, which was done at the introduction to the lesson. The hand does work on the rubber band, and in the stretched position, the rubber band has a certain amount of potential energy. Upon release, that potential energy is converted into kinetic energy. Hence, the rubber band goes flying through the air.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the definition of heat as a form of energy and how it exists in everyday life. They learn about the three types of heat transfer—conduction, convection and radiation—as well as the connection between heat and insulation.
References
Halliday, Resnick, Krane, "Physics." John Wiley & Sons, Inc., 2019
Beichner, Serway, "Physics for Scientists and Engineers." Saunders College Publishing, 2000.
Copyright
© 2013 by Regents of the University of Colorado; original © 2004 Duke UniversityContributors
Adam KemptonSupporting Program
Engineering K-PhD Program, Duke University, Pratt School of EngineeringAcknowledgements
This content was developed by the MUSIC (Math Understanding through Science Integrated with Curriculum) Program in the Pratt School of Engineering at Duke University under National Science Foundation GK-12 grant no. DGE 0338262. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: May 8, 2021



User Comments & Tips