Quick Look
Grade Level: 7 (6-8)
Time Required: 45 minutes
Expendable Cost/Group: US $0.00 The activity may be conducted by using a non-expendable (and highly reusable) BASIC Stamp microcontroller, a temperature probe and other supplies, estimated at $57, or a simple thermometer; see the Materials List for details.
Group Size: 28
Activity Dependency: None
Subject Areas: Measurement, Number and Operations, Physical Science, Physics
NGSS Performance Expectations:

| MS-PS1-4 |

Summary
Students come to see the exponential trend demonstrated through the changing temperatures measured while heating and cooling a beaker of water. This task is accomplished by first appealing to students' real-life heating and cooling experiences, and by showing an example exponential curve. After reviewing the basic principles of heat transfer, students make predictions about the heating and cooling curves of a beaker of tepid water in different environments. During a simple teacher demonstration/experiment, students gather temperature data while a beaker of tepid water cools in an ice water bath, and while it heats up in a hot water bath. They plot the data to create heating and cooling curves, which are recognized as having exponential trends, verifying Newton's result that the change in a sample's temperature is proportional to the difference between the sample's temperature and the temperature of the environment around it. Students apply and explore how their new knowledge may be applied to real-world engineering applications.Engineering Connection
Heat transfer is a broad topic used in many branches of engineering. For example, mechanical engineers who design engines—from steam locomotives to modern internal combustion engines—rely on a detailed understanding of how heat moves through all types of matter. Industrial engineers use heat transfer concepts to design climate control systems for manufacturing facilities, such as foundries or refrigerated food production facilities, which integrate temperature-sensitive human workers with extreme temperature processes. Moreover, heat transfer is so critical to biological engineering that it has spawned the specialty of "bioheat" transfer, which is the study of normal functioning of the cardiovascular system as well as inherently heated treatments such as cryo-surgery and laser-based therapies.
Learning Objectives
After this activity, students should be able to:
- Record data displayed by a temperature probe.
- Plot data points to make graphs.
- Identify a heating or cooling curve as having an exponential trend.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to predict and/or describe phenomena. Alignment agreement: | Gases and liquids are made of molecules or inert atoms that are moving about relative to each other. Alignment agreement: In a liquid, the molecules are constantly in contact with others; in a gas, they are widely spaced except when they happen to collide. In a solid, atoms are closely spaced and may vibrate in position but do not change relative locations.Alignment agreement: The changes of state that occur with variations in temperature or pressure can be described and predicted using these models of matter.Alignment agreement: The term "heat" as used in everyday language refers both to thermal energy (the motion of atoms or molecules within a substance) and the transfer of that thermal energy from one object to another. In science, heat is used only for this second meaning; it refers to the energy transferred due to the temperature difference between two objects.Alignment agreement: The temperature of a system is proportional to the average internal kinetic energy and potential energy per atom or molecule (whichever is the appropriate building block for the system's material). The details of that relationship depend on the type of atom or molecule and the interactions among the atoms in the material. Temperature is not a direct measure of a system's total thermal energy. The total thermal energy (sometimes called the total internal energy) of a system depends jointly on the temperature, the total number of atoms in the system, and the state of the material.Alignment agreement: | Cause and effect relationships may be used to predict phenomena in natural or designed systems. Alignment agreement: |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Model with mathematics.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Construct and interpret scatter plots for bivariate measurement data to investigate patterns of association between two quantities. Describe patterns such as clustering, outliers, positive or negative association, linear association, and nonlinear association.
(Grade
8)
More Details
Do you agree with this alignment?
-
Investigate patterns of association in bivariate data.
(Grade
8)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
New York - Math
-
Reason abstractly and quantitatively.
(Grades
Pre-K -
12)
More Details
Do you agree with this alignment?
-
Model with mathematics.
(Grades
Pre-K -
12)
More Details
Do you agree with this alignment?
-
Construct and interpret scatter plots for bivariate measurement data to investigate patterns of association between two quantities. Describe patterns such as clustering, outliers, positive or negative association, linear association, and nonlinear association.
(Grade
8)
More Details
Do you agree with this alignment?
-
Understand that a function is a rule that assigns to each input exactly one output. The graph of a function is the set of ordered pairs consisting of an input and the corresponding output.
(Grade
8)
More Details
Do you agree with this alignment?
-
Investigate patterns of association in bivariate data.
(Grade
8)
More Details
Do you agree with this alignment?
-
Recognize situations in which a quantity grows or decays by a constant percent rate per unit interval relative to another.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
New York - Science
-
Develop a model that predicts and describes changes in particle motion, temperature, and phase (state) of a substance when thermal energy is added or removed.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Materials List
Each student needs:
To share with the entire class:
- 100 ml beaker
- large heat-proof bowl, at least 6 cm deep
- BASIC Stamp activity kit - Serial + USB (Text v.3.0) (includes the HomeWork Board with a built-in BASIC Stamp 2 microcontroller, LEDs, wires, resistors, capacitors, and USB cable; item code 90005 for $40, available at parallax.com) Alternatively, instead of the BASIC Stamp 2 microcontroller and associated probe and circuitry, use an analog [mercury or other] thermometer.
- Parallax AD592 temperature probe (available at parallax.com; part #28130 for $16)
- 100 Ω resistor (available at radioshack.com; part #271-1311 for $1.19 for five resistors)
- 0.22 μF capacitor (available at digikey.com; part #445-4730-ND for $0.29)
- laptop computer with PBasic software to interface with BASIC Stamp via USB cable (obtain free download of software at https://www.parallax.com/microcontrollers/basic-stamp)
- thermometer, a known one that accurately measures room temperature, for probe calibration
- hot water, tepid water and ice water, ~1 liter each
- Thermometer Program (bs2)
- (optional) projector, to display the temperature probe readings
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/nyu_cooling_activity1] to print or download.Pre-Req Knowledge
Students should have a practical knowledge of temperature and the flow of heat from areas of high temperature to areas of low temperature. Students should be familiar with plotting points on the Cartesian plane, as well as the significance of independent and dependent axes.
Introduction/Motivation
What is heat transfer? Heat transfer is the study of how heat and thermal energy are generated, used and converted. The natural laws governing these phenomena influence almost every aspect of your modern life, and are important to engineers who design all kinds of devices and facilities and systems that we depend upon.
Examples are all around us: cars propelled by internal combustion engines harnessing energy from high-temperature gases; computers that rely on fans to disperse heat built up by electronic processes; many foods that are consumed after cooking to increase the bioavailability of the nutrients; and general public health that is protected through engineered heating processes in food production and sanitation processes.
Mathematically, the shape of a cooling or heating curve is an introduction to nonlinear functions. The way temperature changes in time, which is easy to understand from interaction with the physical world, is an example of physical phenomena that cannot be described by polynomials.
In this activity, you will experience heat transfer, and then apply your knowledge to solve an engineering problem!
Procedure
Before the Activity
- Gather materials and make copies of the Law of Cooling Worksheet.
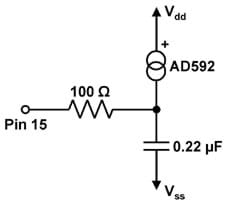
- Build the circuit on the Board of Education diagramed in the schematic in Figure 1 and pictured on the prototyping board in Figure 2.
- Download the PBasic software to a computer and open the attached script, Thermometer Program (bs2).
- Attach the Board of Education to a computer with the USB cable.
- Run the code thermometer.bs2 on the Board of Education. The display will output a reading from the temperature probe.
- Calibrate the temperature probe by using a known (reliable) thermometer and changing the constant "Kal" in thermometer.bs2. To do this, put the temperature probe and the thermometer in a cup of room-temperature water for two minutes and read the temperature from the thermometer. Compare this value with the displayed reading from the temperature probe. If the displayed probe value is larger than the known temperature, decrease the value of Kal and re-run the script. If the displayed value is smaller than the known temperature, increase Kal and re-run script. Iterate this procedure until the temperature probe displays the known temperature.
- Set up the demonstration materials in a location where the class can gather around to watch.
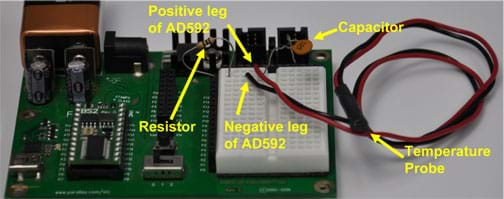
Figure 2: The Parallax Board of Education with built temperature probe circuit. - Obtain ~3 liters of water: 1 liter at room temperature (~20 ºC), 1 liter that is "hot" (~60 ºC), and 1 liter that is "cold" (~10 ºC), and be sure to have ice on hand.
- Prepare the experimental set-up shown in Figure 3 with an ice water bath in the heat-proof bowl. Make the water depth high enough to cover most of the beaker's sides when forced to sit on the bottom of the bowl, but not enough to flood over the sides.
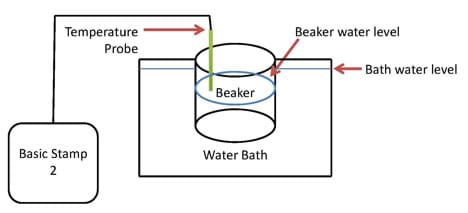
Figure 3: The experimental set-up.
With the Students
- Explore with students their intuition regarding cooling trends, drawing on their personal experiences. For example, ask the students: If you placed a room-temperature can of soda in the refrigerator and waited for it to cool, how would you expect its temperature to change? What kind of trend do you think the temperature would have over time?
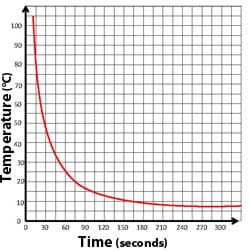
Graph 1. Example exponential curve graph. Notice the changing slope (steepness) of the line. - Present Newton's law of cooling:
- The rate of cooling of a body is proportional to the temperature difference between the body and its ambient environment.
- Draw a graph, explaining that as the temperature of the soda reaches the temperature of the refrigerator, it has less to cool, so the "slope" of the graph is less steep (see Graph 1).
- Distribute a worksheet to each student. Have students predict the response of tepid water in an ice water bath, and prepare the demonstration.
- Place a control volume of room temperature water in a 100 ml beaker and test it in the ice bath. Make sure the beaker can rest on the bottom of the glass bowl and the water bath covers the sides of the beaker but does not flood it (see Figure 4, left).
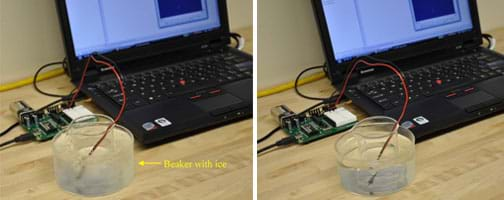
Figure 4: Experimental set-up with ice water bath (left) and hot water bath (right). - Turn on the Basic Stamp and run Thermometer Program (bs2). Designate a student to read aloud the temperature displayed in the debug window at 15-second intervals, either from the computer screen, or from a display, if a projector is available.
- Begin the demonstration by measuring the temperature of the tepid water with the probe. With the probe still in the water, place the beaker in the prepared ice bath and leave it there. While the student announces the temperature at 15-second intervals, have the rest of the class record the temperature data on their worksheets.
- After 5 minutes, stop the program, and have students plot temperature over time on the worksheet grid.
- Discuss the results. Does the trend match the curve predicted by Newton's law of cooling?
- Have students predict the response of tepid water in a hot water bath (see Figure 4, right). Repeat steps 5-7 with a hot water bath and record results. Do the results match the prediction?
- Have students finish answering the questions on the worksheet. Collect the worksheets for grading.
- Conclude by leading a class discussion about engineering examples of applying the principles of heat transfer in the real world, including reviewing students' answers to the worksheet questions.
Vocabulary/Definitions
ambient: Present on all sides; surrounding.
BASIC Stamp: A single-board computer that runs the Parallax PBASIC language interpreter in its microcontroller. The developer's code is stored in an EEPROM, which can also be used for data storage. The PBASIC language has easy-to-use commands for basic I/O, such as turning devices on or off, interfacing with sensors, etc. More advanced commands enable the BASIC Stamp module to interface with other integrated circuits, communicate with each other, and operate in networks. The BASIC Stamp microcontroller has prospered in hobby, lower-volume engineering projects and education due to its ease of use and a wide support base of free application resources. See parallax.com.
heat: Energy transferred from one body to another because of a temperature difference.
Newton's law of cooling: The rate of cooling of a body is proportional to the temperature difference between the body and its ambient environment.
temperature: How hot or cold something is.
Assessment
Pre-Activity Assessment
Cool Experiences: Ask students for examples of where they encounter heating and cooling in their daily lives. Consider the refrigerator. If you placed a room-temperature can of soda in the refrigerator and waited for it to cool, how would you expect the temperature to change? What happens to its temperature over time?
Activity Embedded Assessment
Worksheet: Have students use the attached Law of Cooling Worksheet as they conduct the activity, gathering data, graphing the data and answering questions. Review their answers to gauge their mastery of the subject matter.
Post-Activity Assessment
Heat Transfer in the Real World: Discuss with students a real engineering experience that this experiment models. The following example is one of the worksheet questions; see the answer on the Law of Cooling Worksheet Example Answers.
- Pretend you are an industrial engineer designing a factory to make cookies. You need to design a process to add melted butter to eggs, but you do not want the hot butter to cook the eggs. Butter starts to melt and eggs start to cook around at ~95 degrees Fahrenheit, so if you add a lot of melted butter to the eggs, their temperature will rise and they will cook before they make it to the cookie dough! Can you think of a general idea for how to add a gallon of butter to a gallon of eggs without the eggs getting too hot? What kind of sensors would you need in the system to make sure the eggs do not cook from the butter?
Safety Issues
Water in proximity to electronics always requires caution.
Troubleshooting Tips
Be aware that temperature probes tend to drift, which can lead to difficulty calibrating the probe.
Activity Scaling
- For lower grades, have students discuss Newton's law of cooling and participate in the demonstration using an analog (mercury or other) thermometer.
- For upper grades, have students build the circuit that controls the temperature probe, as well as program the BASIC Stamp using the PBasic software. From a science point of view, have students predict how they think the experiment would change if they used other fluids, such as honey, oil or alcohol, and discuss the concept of specific heat. From a mathematical standpoint, explain more about exponential functions, such as the relationship between an exponential function and its derivative.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students act as food science engineers as they explore and apply their understanding of cooling rate and specific heat capacity by completing two separate, but interconnected, tasks. They collect and graph data to create a mathematical model that represents the cooling rate, and use an exponential ...
References
Giancoli, Douglas C. Physics, Principles with Applications Prentice Hall: Englewood Cliffs, NJ. 1985.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 Polytechnic Institute of New York UniversityContributors
Nicole Abaid; Robert Eckhardt; Karl AbdelnourSupporting Program
AMPS GK-12 Program, Polytechnic Institute of New York UniversityAcknowledgements
This activity was developed by the Applying Mechatronics to Promote Science (AMPS) Program funded by National Science Foundation GK-12 grant no. 0741714. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Additional support was provided by the Central Brooklyn STEM Initiative (CBSI), funded by six philanthropic organizations.
Last modified: January 16, 2020



User Comments & Tips