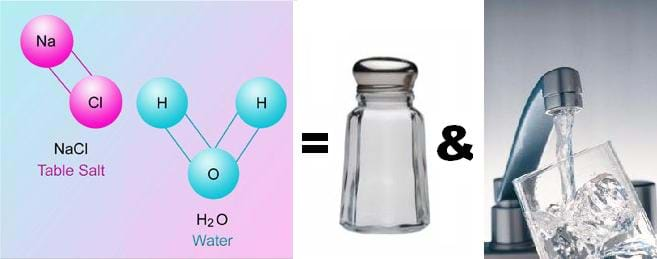
Summary
Through three lessons and their four associated activities, students are introduced to concepts related to mixtures and solutions. Students consider how mixtures and solutions—and atoms and molecules—can influence new technologies developed by engineers. To begin, students explore the fundamentals of atoms and their structures. The building blocks of matter (protons, electrons, neutrons) are covered in detail. The next lesson examines the properties of elements and the periodic table—one method of organization for the elements. The concepts of physical and chemical properties are also reviewed. Finally, the last lesson introduces the properties of mixtures and solutions. A comparison of different mixtures and solutions, their properties and their separation qualities are explored.Engineering Connection
Engineers apply their understanding of the properties of matter to decide what materials to use when creating and designing new things. And, they use their knowledge of mixtures and solutions when designing new synthetic materials for a multitude of purposes. Imagination has no bounds, and as technology advances and researchers learn more about the building blocks of matter, innovative technologies move from imagination to reality.
Engineers understand the properties of metals, whether they may bend or not bend, expand, contract, or withstand forces under certain circumstances—all important factors to consider when designing and creating the devices, structures and human-made objects we depend upon. Furthermore, engineers take advantage of the chemical properties of different elements as they develop mixtures and substances for medicines, materials and cleaning solutions, among the thousands upon thousands of other products that we use in our lives every day.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
See individual lessons and activities for standards alignment.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!Unit Schedule
- Day 1: The Building Blocks of Matter lesson
- Day 2: Gumdrop Atoms activity
- Day 3: Understanding Elements lesson
- Day 4: Engineering and the Periodic Table activity
- Day 5: Properties of Mixtures vs. Solutions: Mix It Up! lesson and Messin' with Mixtures activity
More Curriculum Like This

Students examine the periodic table and the properties of elements. They learn the basic definition of an element and the 18 elements that compose most of the matter in the universe. The periodic table is described as one method of organization for the elements.

Students learn about atoms and their structure (protons, electrons, neutrons) — the building blocks of matter. They see how scientific discoveries about atoms and molecules influence new technologies developed by engineers.

Students use gumdrops and toothpicks to make lithium atom models. Using these models, they investigate the makeup of atoms, including their relative size.

Students learn how to classify materials as mixtures, elements or compounds and identify the properties of each type. The concept of separation of mixtures is also introduced since nearly every element or compound is found naturally in an impure state such as a mixture of two or more substances, and...
Copyright
© 2009 by Regents of the University of ColoradoContributors
See individual lessons and activities.Supporting Program
Integrated Teaching and Learning Program, College of Engineering and Applied Science, University of Colorado BoulderAcknowledgements
The contents of these digital library curricula were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education, and the National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the U.S. Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: February 15, 2018





User Comments & Tips