Quick Look
Grade Level: 6 (5-7)
Time Required: 15 minutes
Lesson Dependency: None
Subject Areas: Chemistry, Physical Science
NGSS Performance Expectations:

| MS-PS1-1 |
Summary
Students examine the periodic table and the properties of elements. They learn the basic definition of an element and the 18 elements that compose most of the matter in the universe. The periodic table is described as one method of organization for the elements. The concepts of physical and chemical properties are also reviewed.
Engineering Connection
Engineers use the properties of matter to decide what materials to use when creating and building things. For example, metals have certain properties that allow them to bend or not bend, to expand and contract, and to hold certain amounts of weight. Engineers also use the chemical properties of different elements to develop mixtures and substances for new medicines and products.
Learning Objectives
- Distinguish between physical and chemical properties.
- Describe the arrangement of elements in the periodic table of elements.
- Describe how engineers use knowledge of the physical and chemical properties of elements when choosing materials for products.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to predict and/or describe phenomena. Alignment agreement: | Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. Alignment agreement: Solids may be formed from molecules, or they may be extended structures with repeating subunitsAlignment agreement: | Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Develop an evidence based scientific explanation of the atomic model as the foundation for all chemistry
(Grade
6)
More Details
Do you agree with this alignment?
Pre-Req Knowledge
Students should know the basic properties and structure of the atom.
Introduction/Motivation
We want to learn more about the properties of a substance or object, so we can determine what tools and materials to use when designing and creating things as engineers. For example, the properties of crayons and markers are different, yet they both produced color. What are the properties of a crayon? (Write answers on the board. Possible answers: hard, dry, waxy texture, melts, different colors, etc.) Okay, what are the properties of a marker? (Write answers on the board in a new list. Possible answers: smooth tip, sweet smelling, sour smelling, different colors, wet, etc.) We decide whether we are going to use a crayon or a marker when coloring a picture, depending on the end result that we want. The end result is also how engineers choose which materials they will use to design something.
The properties of a substance can be separated into two categories: physical and chemical. Physical properties can be observed in an object, such as size, shape, color, even the boiling point. Chemical properties are the ability to combine with another substance and make a new substance, such as when water and iron combine to make rust. Are the properties we have listed physical properties or chemical properties? They are physical properties because they do not change into something else.
Both physical and chemical properties are based on the behaviors of the elements that make up the object. An element is something that is made of the same kind of atoms, which are made up of protons, electrons and neutrons. For example, the element lead is made up of all lead atoms, nothing else. While more than 100 elements exist, 18 of then make up most of the known universe. Some of these 18 elements you may have already heard of: hydrogen, helium, carbon, oxygen, nitrogen, sodium, magnesium, phosphorus and sulfur. Can you think of anything that has one of those elements or a combination of those elements in it? Well, water and air are both made of some of the elements that we just listed. In fact, hydrogen makes up 90% of all matter in the universe. Carbon is an element found in all things that are living.
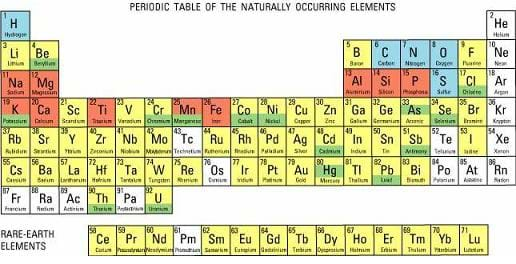
The elements can be arranged in many ways; however, the most common way is in what we call the periodic table of elements. Elements are arranged in the periodic table based on their properties (both physical and chemical, although mostly chemical). The periodic table organizes elements based on two major things: the number of shells an element has for its electrons determines the rows (periods) where the element is placed, and the number of electrons in the outer most shell of the element determines the column (group) where the element is placed. The period or group that the element is placed in tells us a lot about the properties of that element. We are going to learn more about that today.
Engineers take into consideration materials' physical and chemical properties when designing almost anything, including buildings and new medicines. Engineers want to design buildings that are strong. They also want to design medicine to be both safe and effective. For example, metals have certain properties that allow them to bend or not bend, to expand and contract, and to hold certain amounts of weight. If engineers were designing a bridge over a river, they would want to choose a metal that would be strong and somewhat flexible. However, they would make sure not to choose a metal that expands or contracts too much in different types of weather. That's because if a bridge grew or shrank too much in size, it could collapse, possibly causing injury to people and damage to surrounding structures.
Lesson Background and Concepts for Teachers
Matter is often defined by physical properties. Physical properties are attributes that can be observed in an object. Some examples of properties include color, odor, size, weight, texture, buoyancy, boiling point, melting point, freezing point and shape. Matter is also defined by chemical properties. These are attributes that affect the chemical state of matter. For example, one chemical property may be the ability to combine with another element in a chemical reaction.

Elements
An element is a substance that cannot be broken down by chemical reactions. The smallest particle of an element is the atom, which is made of protons, electrons and neutrons. For example, the pure element gold is made of gold atoms and nothing else. If you add or subtract a proton from the nucleus of an atom, you create an entirely new element. For instance, if you subtract one proton from an atom of gold, you have platinum. If you add or subtract a neutron from the nucleus of an atom, then a new element is not created, instead you create a new isotope. Carbon-14 is an isotope of carbon, has two extra neutrons, and is radioactive and used for carbon dating.
More than 100 elements have been discovered or created, but this lesson focuses on the first 18, which compose most of the matter in the universe. The lightest elements are hydrogen and helium. Hydrogen is also thought to be the first element that appeared on Earth.
Currently, more than 20 elements are not derived naturally, but have been artificially synthesized by humans. Elements can be categorized in many ways, including name, symbol, atomic number, atomic mass, boiling point and melting point; however, the most common chart of the elements is the periodic table, which organizes the elements by chemical properties.
Figure 1 lists the first 18 elements in the periodic table.
The Periodic Table
The periodic table was first derived in 1869 by chemist Dimitri Mendeleev. It is a chart of the known elements that is organized like a big grid with rows and columns. The elements are placed in rows, called periods, because of their similar characteristics. All of the elements in a period have the same number of shells for their electrons. Elements in the top row have one shell, second row two shells and so on down the table for a maximum of seven. Students can learn more about the periodic table with the associated activity Engineering and the Periodic Table where they will as well work as animation engineers to create a superhero character based on one of the elements.
The elements are also placed in columns, or groups, that have the same number of electrons in their outer shells. Elements in the first column have one electron in their outer shells, second column two electrons and so on. Some special groups exist on the periodic table, including the noble gases in group 18 (or 8, if not counting the transition elements) that have full outer valence shells. This means that they do not need any electrons to complete the shell and therefore are highly un-reactive. Also, the halogens in group 17 (or 7) are all missing one electron to fill their outer shells. All of these elements bond with hydrogen to form acids, such as hydrochloric acid.
Associated Activities
- Engineering and the Periodic Table - Students learn about the periodic table and the pervasiveness of the first 20 elements in our everyday, engineered world by playing a team game. They also work as animation engineers to create a superhero character based on one of the elements.
Lesson Closure
Today, we talked about the physical and chemical properties of elements and how these are important to engineers. Who remembers what the difference is between physical and chemical properties? (Listen to student responses.) Well, physical properties are those that can be observed in objects, such as size, shape, color and even boiling point or density. Chemical properties deal with the chemical state of objects, such as their ability to combine with other substances in reactions. We also learned that elements are arranged in the periodic table based on physical and chemical properties (although mostly chemical). The way the elements are arranged in rows and columns on the periodic table tells us more about them.
It is important that engineers use their knowledge about the properties of elements and materials when creating new technologies. Let's pick one item in this classroom and list its properties. Imagine what that object would be like if it were made using entirely different materials. That is what engineers might do when creating a design that improves on an existing technology.
Vocabulary/Definitions
chemical property: A characteristic of matter that affects the chemical structure of matter.
element: Substance that cannot be broken down by chemical reactions.
isotope: Any atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with different number of neutrons in the nucleus.
physical property: A characteristic of matter, such as boiling point, melting point, freezing point, density, color or smell.
Assessment
Pre-Lesson Assessment
Discussion Question: Ask the class some questions to get students to think about the upcoming lesson. After soliciting answers, explain that these questions will be answered during the lesson.
- What is an atom? (Answer: A fundamental building block of matter. Every atom consists of negatively-charged electrons and a nucleus made of positively-charged protons and neutrally-charged [neither positively- nor negatively-charged] neutrons.)
- Name one atom you might find in this classroom.
Define It: In small groups, have students engage in open discussion. Remind them that all ideas should be respectfully heard. Ask the students:
- How would you describe a "property" of an object? (Answers will vary. Possible answers include: smells, odorless, sticky, large, small, red, etc.)
Post-Introduction Assessment
Vocabulary: Ask students to write down the following vocabulary words on a sheet of paper or in their science journals and then develop their own definitions of these words based on what they have learned so far. Terms to use:
- element (A substance that cannot be broken down by chemical reactions.)
- physical property (A characteristic of matter, such as boiling point, melting point, freezing point, density, color or smell.)
- chemical property (a characteristic of matters that affects the chemical structure of matter.)
Lesson Summary Assessment
Engineering Redesign Similar to the lesson closure, have students (acting as engineers) each pick an item in the classroom (whiteboard, chair, table, etc), and design an item that can replace it using different materials than that of which the item is currently made. Have students list the benefits of their re-design and decide if their replacements are better than the originals. Remind students that engineers often redesign items that already exist to improve them.
Lesson Extension Activities
Research: Assign each student a different element from the periodic table (preferably from the first 18), and have them research where that element is present (ithat is, what objects in the classroom are composed of that element, etc.). Have students count how many rows down in the periodic table their elements are to see how many shells the elements have for their electrons. Have students count the columns (from the left in the table) of where their elements are located in the table to see how many electrons are in the outer shell of the elements.
Other Element Games: Have students play the element games at Jefferson Lab: http://education.jlab.org/indexpages/elementgames.php
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about atoms and their structure (protons, electrons, neutrons) — the building blocks of matter. They see how scientific discoveries about atoms and molecules influence new technologies developed by engineers.

Students use gumdrops and toothpicks to make lithium atom models. Using these models, they investigate the makeup of atoms, including their relative size.

Students learn how to classify materials as mixtures, elements or compounds and identify the properties of each type. The concept of separation of mixtures is also introduced since nearly every element or compound is found naturally in an impure state such as a mixture of two or more substances, and...

Students are introduced to the concept of energy cycles by learning about the carbon cycle. They learn how carbon atoms travel through the geological (ancient) carbon cycle and the biological/physical carbon cycle.
References
Pearson Education, Inc., Family Education Network, "Proton Don," accessed August 31, 2006. http://www3.funbrain.com/cgi-bin/pt.cgi?A1=s&A2=1&ACOMMON=1&submit=Play+Proton+Don
Thomas Jefferson National Accelerator Facility - Office of Science Education, Science Education, Games & Puzzles, "Element Games," accessed August 31, 2006. http://education.jlab.org/indexpages/elementgames.html
U.S. Department of the Interior, U.S. Geological Survey, Publications Warehouse, August 16, 2006, accessed September 12, 2006. http://pubs.usgs.gov/circ/c1143/html/fig9.jpg
Winter, Mark, The University of Sheffield, "WebElementsTM Periodic Table Scholar Edition," accessed September 12, 2006.
Copyright
© 2006 by Regents of the University of Colorado.Contributors
Brian Kay; Daria Kotys-Schwartz; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: August 16, 2024




User Comments & Tips