Quick Look
Grade Level: 6 (5-7)
Time Required: 1 hour
Expendable Cost/Group: US $0.00
Group Size: 2
Activity Dependency: None
Subject Areas: Chemistry, Physical Science
NGSS Performance Expectations:

| MS-PS1-1 |
Summary
Students learn about the periodic table and how pervasive the elements are in our daily lives. After reviewing the table organization and facts about the first 20 elements, they play an element identification game. They also learn that engineers incorporate these elements into the design of new products and processes. Acting as computer and animation engineers, students creatively express their new knowledge by creating a superhero character based on of the elements they now know so well. They will then pair with another superhero and create a dynamic duo out of the two elements, which will represent a molecule.
Engineering Connection
Information in the periodic table of the elements helps engineers in all disciplines, because they use elements in all facets of materials design. Exploiting the characteristics of the various elements helps engineers design stronger bridges, lighter airplanes, non-corrosive buildings, as well as agriculture, food, drinking water and medical products. Since everything known to humans is composed of these elements, everything that engineers create uses this knowledge.
Learning Objectives
After this activity, students should be able to:
- Identify three elements and several of their characteristics.
- Describe how engineers always use their knowledge about element properties when designing and creating virtually everything we see around us.
- Use the superhero analogy to make models of both atoms and molecules.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to predict and/or describe phenomena. Alignment agreement: | Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. Alignment agreement: Solids may be formed from molecules, or they may be extended structures with repeating subunitsAlignment agreement: | Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Develop an evidence based scientific explanation of the atomic model as the foundation for all chemistry
(Grade
6)
More Details
Do you agree with this alignment?
-
All matter is made of atoms, which are far too small to see directly through a light microscope. Elements have unique atoms and thus, unique properties. Atoms themselves are made of even smaller particles
(Grade
6)
More Details
Do you agree with this alignment?
Materials List
For Part 1: Engineering the Elements Matching Game, the teacher needs:
- Elements Matching Game Images PowerPoint file
- A computer projector or overhead projector to show the PowerPoint slides
For Part 1: Engineering the Elements Matching Game, each group needs:
- 1 set of Elements Matching Game Cards
For Part 2: Designing Element Superheroes, teacher needs:
- 1 set of either Elements Matching Game Cards (okay to re-use from Part 1) or Mystery Elements Cards (this option requires students to do more research)
For Part 2: Designing Element Superheroes and Dynamic Duos, each group needs:
- Student access to information about all the elements (such as physical science books or the Internet)
- Paper
- Colored pencils or markers
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_mix_lesson2_activity1] to print or download.Pre-Req Knowledge
A basic understanding of the periodic table of the elements. A basic understanding of the structure of an atom is helpful, as presented in the The Fundamental Building Blocks of Matter lesson in the Mixtures & Solutions unit.
Introduction/Motivation
Let's make a list of all the elements we can think of and write them on the board (or on an overhead transparency). Remember that the elements in the periodic table cannot be further broken down to form a different element. Think of elements as the most basic building blocks. These building blocks are what combine to create everything we see around us. (If some students suggest compounds [such as water or air], clarify the difference between elements and compounds [water is a compound of hydrogen and oxygen elements; air is mostly nitrogen and oxygen].)
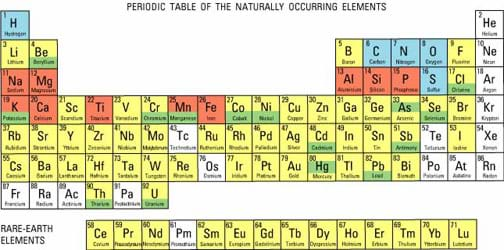
Who remembers that the periodic table organizes the elements based on their properties? Today let's learn about some of those properties. (See Figure 2. Show the periodic table, poster size or via overhead projector using the attached Periodic Table Visual Aid or from the Internet using the dynamic periodic table at http://www.dayah.com/periodic/.) Let's find the elements you already know. (Point out the locations of all the elements in the student-generated list.)
The periodic table tells us a lot of information about the elements. First of all, elements are arranged in different groups (vertical) and periods (horizontal). So, the elements with similar properties are grouped together. The periodic table has several categories, such as: non-metals, halogens, noble gases, metalloids, alkali metals, alkaline earth metals and poor metals. What else can we learn by looking at the periodic table? (Possible answers: element names, element abbreviations, atomic numbers, numbers of protons, rare earth elements, etc.) What can we learn from how they are arranged in the table? (They are arranged by their number of protons, or atomic number.)
Why do you think engineers must understand the periodic table? (Answer: Understanding the elements of the periodic table and how they interact with each other is important for engineers because they work with all types of materials. Knowledge of the characteristics of the various elements helps them design stronger bridges, lighter airplanes, non-corrosive buildings, the buttons on your toys and games, as well as food and medical applications.) It is essential for engineers to understand the properties of the different elements so that they know what to expect or look for when designing something new. Engineers are always trying to improve things — like airplanes, air conditioning systems, computers or cell phones. Better designs often include an improvement in the materials used, and materials are made of elements, or compounds of one or more elements. An engineer keeps the different element properties in mind when designing.
Today we are going to learn more about the properties of elements in the periodic table. We will learn about the engineering applications of many of the elements. With this information, we will work as computer and animation engineers who are designing a superhero who has similar characteristics to an element. Then we will make a periodic table of superheroes that could be used in a TV show or computer game!
We will then discover the nature of atoms interacting as molecules by forming pairs of elemental characters to make superhero groups and describe the combined behavior of our two different elements.
Procedure
Background
Looking at the periodic table in a science book (or Figure 2 or the attached Periodic Table Visual Aid or the dynamic periodic table at https://ptable.com/#Properties), the vertical columns are referred to as groups while the horizontal rows are known as periods. From left to right, the groups are classified as alkali metals (group 1), alkaline earth metals (group 2), transition metals (groups 3-12), poor metals/metalloids/non metals (groups 13-16), halogens (group 17), and noble gases (group 18). In most periodic tables, the different groups are labeled with different colors. In addition to the main groups and rows, two mini-periods are often separated from the main table and placed below it. The lanthanide and actinide series are known as rare earth elements. All of the elements occur either naturally in that form, arise from the decay of those natural elements, or are synthetic (human-made). Depending on the age of the table, the number of synthetic elements may vary.
Elements are arranged based on their number of protons, which is commonly referred to as their atomic number. They increase in number from left to right and from top to bottom. In addition, the order corresponds to the atomic mass of the element as well (from smallest to largest) for most of the elements. Elements are further arranged based on common properties between elements, such as electron configurations and electronegativity. In this activity, students learn about the first 20 elements on the periodic table, all of which are present in our daily lives.
When two or more atoms join together they create a molecule. For example, one molecule of water is made up of two hydrogen atoms and one oxygen atom.
Before the Activity
- Gather materials.
- For Part 1, prepare a computer projector or overhead projector to show the attached Elements Matching Game Images Power Point presentation to the class. Also, print and cut apart three sets of the attached Elements Matching Game Cards. Shuffle each set.
- For Part 2, either re-use one of the Part 1 sets of Elements Matching Game Cards, or print out and cut apart one set of the attached Mystery Elements Cards. Student teams will each blindly choose a card from the set you provide. The first set provides property and characteristic information on the first 20 elements. The second set provides more of a challenge; its cards provide property and characteristic information on the first 30 elements without identifying the element names, so students must first identify "their" element before proceeding with the activity.
With the Students: Part 1 — Engineering the Elements Matching Game
- To conduct the Elements Matching Game, divide the class into three teams.
- Give each team a set of element game cards to distribute evenly among their teammates.
- Explain the activity to the students.
- To begin, the teacher shows pictures and clues of 20 unidentified elements (using the attached Elements Matching Game Images PowerPoint file). (Answers may be found on the last slide.)
- Students look at their game cards until someone discovers that they are holding the card that matches the unknown element.
- The first person who raises their hand, (and correctly) declares that they have the matching element, scores a point for their team. (Each team has the same set of cards, so teams are competing to identify each element first).
- The student who correctly identifies the element reads the rest of their card to the class.
- The teacher shows the next slide (image of another element), and the game continues.
- After 20 elements are matched, the team with the most points is declared the winner.
- Reiterate the point that the elements combine together to create many different compounds that are used by engineers. Ask students if they re surprised to learn how many engineering applications the elements have when put together in different combinations. For example, engineers use lithium in cell phone batteries and aircraft parts.
With the Students: Part 2 — Designing Element Superheroes
- Explain to students that some engineers are involved in graphic design, special effects and computer animation. They develop handheld electronic and computer games as well as the animated movies and TV shows that students might watch. Often, these graphics and animations are designed for educational purposes — to teach viewers about a school subject. Today, students act as computer and animation engineers and develop a new educational character based on the elements in the periodic table.
- Divide the class into teams of two students each. Assign one element per team by having them randomly choose an element from either the Elements Matching Game Cards (the first 20 elements, identified) or the Mystery Elements Cards.(the first 30 elements, unidentified) (If using the mystery cards, the students must conduct research to determine the name of their element. Provide resources such as physical science books, periodic table handouts or Internet access. Students may need assistance for some of the more unfamiliar elements.)
- After each team has an element, ask them to design a superhero based on the characteristics of that element to use for a new educational animation series. Before designing, direct them to choose a specific audience for their character (elementary, middle or high school students), and keep that audience in mind when determining the nature, aesthetics (the looks) and super power of their character. For example, think of the various animated characters that are popular today with younger kids (perhaps Dora the Explorer or Sponge Bob), compared to those popular with teens (perhaps football/skateboard game characters or Japanese anime). What are the differences in the visual look and nature of these characters? (Perhaps colors [bright vs. dark], shapes [simple vs. complex], nature [childlike vs. mature], etc.) The point is to create something that appeals to your target audience.
- Direct students to refer to the properties on their cards, and design their superhero to have similar characteristics. The superhero's main power should be related to an item on the information card. Guide students to brainstorm together to come up with creative ideas. If it helps to generate ideas, show the attached Element Superhero Example (also Figure 1). Remind students of the brainstorming tips used by engineers:
- No negative comments allowed.
- Encourage wild ideas.
- Record all ideas.
- Build on the ideas of others.
- Stay focused on the topic.
- Allow only one conversation at a time.
- Remind students that each group must come up with a name for the superhero that relates to the name of the element.
- Have students draw their superhero (as time permits). Each team drawing should include the element's symbol and atomic number, as well as a short description of the hero's powers and properties.
- Once the students have finished or made reasonable progress on their super heroes, remind them that not all superheroes work alone and have them brainstorm different pairs or groups of superheroes.
- Now pair each group of students with another group of students. Have them come up with a new super group. They should be thinking about what strengths and weaknesses each elemental superhero brings to the group and what the group is best at, based on the individual strengths of the elements.
- Have each team make a quick "engineering design" presentation of their first individual elements and superheroes and then their dynamic due to the class. See the Assessment section for suggested presentation requirements.
- Arrange the superhero element drawings on a wall by having the students arrange them to mimic their relative periodic table locations.
- Conclude by having the entire class participate in a Human Periodic Table, as described in the Assessment section.
Vocabulary/Definitions
atom: The basic unit of matter; the smallest unit of an element, having all the characteristics of that element; consists of negatively-charged electrons and a positively-charged center called a nucleus.
atomic number: The number of positive charges (or protons) in the nucleus of an atom of a given element, and therefore also the number of electrons normally surrounding the nucleus.
brainstorming: A method of shared problem solving in which all members of a group quickly and spontaneously contribute many ideas.
compound: (chemistry) A pure substance composed of two or more elements whose composition is constant.
electron: Particle with a negative charge orbiting the nucleus of an atom.
element: (chemistry) A substance that cannot be separated into a simpler substance by chemical means.
engineer: A person who applies their understanding of science and math to creating things for the benefit of humanity and our world.
Materials science: The study of the characteristics and uses of various materials, such as glass, plastics and metals.
molecule: A group of atoms bonded together.
nucleus: Dense, central core of an atom (made of protons and neutrons).
periodic table: (chemistry) A table in which the chemical elements are arranged in order of increasing atomic number. Elements with similar properties are arranged in the same column (called a group), and elements with the same number of electron shells are arranged in the same row (called a period).
proton: Particle in the nucleus of an atom with a positive charge. Elements are arranged in the periodic table based on their number of protons (or atomic number).
synthetic element: (chemistry) An element too unstable to be found naturally on Earth.
Assessment
Pre-Activity Assessment
Information Pooling: Ask the class to think of all the elements they know. Compile a list on an overhead projector transparency or the classroom chalk board as the students make suggestions. If some students suggest compounds (such as water or air), clarify the difference between elements and compounds. When no more suggestions are forthcoming, bring out the periodic table, and point out the locations of all the elements suggested by the students.
Activity Embedded Assessment
Pairs Check: After student teams create their superhero character from their element card, have them check with another group to verify that they have the correct information included in their design sketch.
Post-Activity Assessment
Engineering Design Presentations: Have each team present their design of an element superhero. Require the presentations to include: the name of the element, the element clues that were given, specifically how the element was identified, the chemical symbol, the atomic number, the name of the superhero, how the superhero's look relates to the element, how the superhero's powers relate to the element, the audience for the character, and how they designed it for that audience, and a drawing of the superhero.
Human Periodic Table: Ask students to clear an area in the classroom (move desks aside or go outside) and arrange themselves like the common periodic table. As time permits, go around (as they are arranged) and ask them to explain the logic of their element position in the table, using what they learned during the activity.
Activity Extensions
Extra Fun Facts: If students have access to more science books and/or the Internet, have them, in addition to determining the name of their element, find out another fun fact about the element. At the end of the activity, in their class presentation or while they are describing their position in the Human Periodic Table (see Assessment section), have teams share this fact with the class.
Complete the Table: Make a full superhero periodic table by assigning the rest of the elements to the students. Have them research the elements enough to design a superhero with similar characteristics. Then hang these on the wall with the original 20 element superheroes.
Activity Scaling
- For lower grades, add the chemical symbol and/or atomic number to each element card to provide an easy clue.
- For more advanced, modify the element cards by removing the first clue from each one (this is the easiest clue).
Additional Multimedia Support
A great online resource is the "dynamic periodic table" at Michael Dayah's website. It provides colorful, interactive and current information on series, properties, electrons, isotopes, element characteristics (and more), and in the language of your choice. If possible, project it on your classroom wall from a computer/Internet connection as you discuss the periodic table and elements with the class, Or, use the PDF letter and legal sizes for color handouts. Click on "About" to fully explore the capabilities of this resource. See: https://ptable.com/#Properties.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students examine the periodic table and the properties of elements. They learn the basic definition of an element and the 18 elements that compose most of the matter in the universe. The periodic table is described as one method of organization for the elements.

Students learn how to classify materials as mixtures, elements or compounds and identify the properties of each type. The concept of separation of mixtures is also introduced since nearly every element or compound is found naturally in an impure state such as a mixture of two or more substances, and...
References
Dictionary.com. Lexico Publishing Group, LLC., http://www.dictionary.com, accessed July 24, 2007. (Source of some vocabulary definitions, with some adaptation)
Periodic Table of the Naturally Occurring Elements. Publications Warehouse, U.S. Geological Survey Circular 1143, Version 1.0, USGS Online Publications. Accessed July 24, 2007. http://pubs.usgs.gov/
Copyright
© 2006 by Regents of the University of Colorado.Contributors
Megan Podlogar; Lauren Cooper; Brian Kay; Malinda Schaefer Zarske; Denise W. CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education, and National Science Foundation GK-12 grant no 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: September 19, 2023




User Comments & Tips