Quick Look
Grade Level: 7 (6-9)
Time Required: 45 minutes
Expendable Cost/Group: US $8.00 You can use narrower beakers to reduce materials used, and thus cost per group.
Group Size: 2
Activity Dependency: None
Subject Areas: Physical Science

Summary
Students explore the densities and viscosities of fluids as they create a colorful 'rainbow' using household liquids. While letting the fluids in the rainbow settle, students conduct 'The Great Viscosity Race,' another short experiment that illustrates the difference between viscosity and density. Later, students record the density rainbow with sketches and/or photography.Engineering Connection
Fluid properties are important in the design of any device that interacts with a fluid, either liquid or gas. Knowledge about density inspired engineers to build ships, inner tubes, surfboards, buoys and other flotation devices, while the understanding of viscosity allows engineers to build not only ships that float, but also ships that can travel fast, and airplanes that can fly at supersonic velocities. Density and viscosity are also important properties that engineers consider in the engines they design for automobiles and power plants.
Learning Objectives
After this activity, students should be able to:
- Define density and viscosity.
- Compare and contrast density and viscosity.
- Represent density and viscosity by their equations and define all parts of each equation.
- Notice that viscosity is dependent on density, but can be smaller or greater based on temperature, so they are not proportional.
- Explain why it is useful to know how to calculate density and viscosity.
- Give examples of how engineers use this information, such as in flight (real world examples).
- See that visualizing a fluid flow can both show the physics that are occurring, and be beautiful.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
Common Core State Standards - Math
-
Decide whether two quantities are in a proportional relationship, e.g., by testing for equivalent ratios in a table or graphing on a coordinate plane and observing whether the graph is a straight line through the origin.
(Grade
7)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Develop and design a scientific investigation to separate the components of a mixture
(Grade
7)
More Details
Do you agree with this alignment?
-
Predict and evaluate the movement of an object by examining the forces applied to it
(Grade
8)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- a box of food coloring
- 1 rectangular glass container or vase (no smaller than 15 cm x 10 cm x 8 cm or 6 in x 4 in x 3 in; available at hobby or craft supply stores)
- 5 small disposable cups (preferably transparent)
- marker (to label cup contents)
- ¼ cup (59 ml) isopropyl alcohol
- ¼ cup (59 ml) shampoo (Pantene Pro-V recommended)
- ¼ cup (59 ml) strawberry syrup (Hershe's brand syrup recommended
- ¼ cup (59 ml) corn syrup
- ¼ cup (59 ml) cooking oil
- ½ cup (118 ml) ketchup
- ½ cup (118 ml) chocolate syrup
- 1 clear 8 oz (.24 l) drinking glass
- 1 spoon or mixing rod (or a small supply of plastic spoons or stirrers)
- 3 sheets white paper
- tape (to affix white paper securely to wall and tabletop)
- pencils (for completing the worksheets
- Student Instructions Handout, one per group
- Density Rainbow and the Great Viscosity Race Worksheet one per person
Note: Narrower beakers can be used, and amounts reduced proportionally. Try to maintain about a one-inch depth for each layer.
Note: All materials can be poured down the drain for disposal.
To share with the entire class (optional):
- camera, of any type
- black cardboard or fabric for photograph background. 2 ft X 2 ft (or .6 m x .6 m)
- 60 Watt clip-type light or equivalent
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_flow_activity2] to print or download.Introduction/Motivation
Two properties of fluids that are often easily confused are density and viscosity. What is density? What is viscosity? Density is the measure of mass per unit volume, or how heavy a fluid is. Viscosity is a measure of how stiff a fluid is, or how easily, or quickly, it flows.
It is easy to measure the density for liquids; just see how much it weighs. What is interesting is when fluids of different densities get together; the densest fluid sinks to the bottom and the less dense fluid stays above it. You may have seen this before if you have noticed how motor oil floats on top of water in a puddle.
Within a single fluid such as air or liquids, the cooler the fluid, the denser it gets. The idea that 'heat rises' comes from the fact that hot gases are less dense than their cooler neighbors, so up they go. In the atmosphere, this effect is responsible for cumulus clouds, which form from rising heated air. In the ocean, salty water is denser than fresh water. The dense, saltier water stays deeper in the ocean until other forces make it move. On a grand scale, the ocean is denser than the atmosphere, so it stays below the air around us. Imagine if it was the other way around!
When dense fluids stay below less dense fluids, we end up with stratified fluids. In the density rainbow activity, we will layer liquids of different densities and colors. The densest ones go in first, on the bottom of the glass. Stratified fluids are pretty stable, so the rainbow we create will last until you stir it up. If you try to pour a heavier fluid on top, your combination becomes unstable, and the new, denser fluid sinks to the bottom. This is called the Rayleigh-Taylor instability.
Viscosity measures the resistance of a fluid to flow. For example, it takes longer to pour corn syrup out of a bottle than to pour water from a similar bottle. This shows that corn syrup is more viscous than water. Now, corn syrup is both denser and more viscous than water, but viscosity and density do not always go together. Absolute viscosity is independent of density, but because dense fluids usually are more viscous, the two properties are often confused. In the Great Viscosity Race, we will compare the speeds of two fluids, and then compare their densities. Will the less dense fluid win by being less viscous too? Let's find out!
Procedure
Before the Activity
- Divide the class into teams of two or three students each.
- Gather materials and make copies of the Student Instructions Handout, one per group, and the Density Rainbow and the Great Viscosity Race Worksheet, one per person.
- Set out the chocolate syrup and ketchup to warm to room temperature.
- Prepare one station or table for each group, as described below.
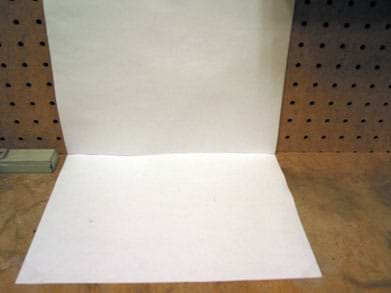
- To set up the stations, choose table top surfaces that are cleanable. For the best observations, position the back of each table against a wall.
- On the wall behind each station, tape a piece of white paper so that one edge of the paper aligns with the edge of the tabletop (see Figure 2).
- Tape another piece of white paper on the table so that the edges of this paper align with the one already taped to the wall (see Figure 2).
- Set 5 disposable cups at each station and fill each one quarter full with a different fluid (corn syrup, strawberry syrup, shampoo, cooking oil and isopropyl alcohol). Label each cup with the fluid it contains.
- Place glass container, food coloring and a spoon at each station.
With the Students
Part 1: The Density Rainbow
- Dye the corn syrup, alcohol and shampoo different colors by dropping 1 or 2 drops of food coloring into each cup. Make sure not to dye a fluid pure red because the strawberry syrup is already that color. You can also experiment with mixing different colors of food coloring to obtain colors such as purple and orange by dropping in drops of two different colors into a fluid. It is important that no more than 2 drops of blue are dropped into the fluid because too much food coloring makes a very dense color which, in turn, looks black. Record on your worksheet what color is used for each fluid.
- Stir each dyed fluid thoroughly with the spoon/mixing rod. (Remember to rinse the spoon or mixing rod so the food colors do not mix, or use disposable plastic spoons or stirrers.)
- Answer questions 2-3 on your worksheet.
- On your worksheet, predict which fluid will be the densest, and which will be the least dense.
- As a team, decide on the order of fluids, from the most to least dense. Then pour what you think is the most dense fluid into the glass container first, being careful not to drip any on the sides of the container. Slowly pour the next densest fluid on top of the previously poured fluid. If poured slowly, no mixing should happen between them and the layers will remain visible. If the container is very tall, use a spoon to ease the fall (see Figure 3). Although some mixing will probably happen, your fluids will settle after a while (see Figure 4). Keep pouring in the fluids in order of density (the most dense at the bottom, and the least dense at the top).
- When you poured in the fluids, did they stay in the order you poured them? Or did some that you thought were denser move up a layer? Why did the fluids set in that order? Answer question 5 on your worksheet.
- Give students numbers for mass and volume of each fluid and allow them to calculate the actual densities so as to see the results in a different light.
- The rainbow takes at least five minutes to settle out and have distinguishable layers (see Figure 7). After Part 3, all materials can be poured down the drain for disposal.


Part 2: The Great Viscosity Race
- Our two racing fluids are chocolate syrup and ketchup.
- First, see which is denser. Pour about 1/3 cup (78 ml) of chocolate syrup into the clear 8 oz (.24 l) drinking glass.
- Pour ketchup into the glass, above the syrup. If you pour it in slowly, it should stay above the syrup, showing that the ketchup is less dense than the syrup.
- Given these two fluids, calculate the density of each given the specified measurements.
- Answer questions 6-8 on your worksheet.
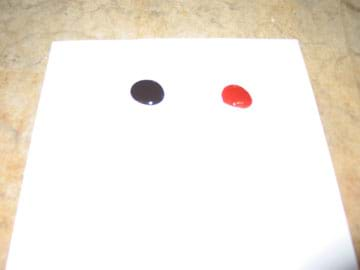
- Place a white piece of paper on the table, and at one end of the paper, place a small spoonful of ketchup next to a small spoonful of syrup. Make sure that they are not touching (see Figure 5).
- Based on your observations so far, which liquid do you think will flow faster down the sheet? Answer question 9 on your worksheet.
- Tilt the piece of paper to a vertical orientation. Observe which fluid flows faster down the paper (see Figure 6). The winner is the less viscous fluid.

- Compare the ranking of viscosity to the ranking of density for the ketchup and chocolate syrup. Was the least dense also the least viscous? Answer questions 10-12 on your worksheet. After Part 3, all materials can be poured down the drain for disposal.
Part 3 (optional): The Density Rainbow Revisited
- Return to your density rainbow container and observe how the fluids have separated (see Figures 1 and 7).
- Position a black background behind the density rainbow.
- Experiment with the clip light and camera position so that the light glows through the density rainbow while the rest of the image is dark. Adjust your camera to make the rainbow fill the composition. Use the camera to capture stunning pictures of the rainbow.
- Clean-up: All materials from the rainbow and race can be poured down the drain for disposal.

Vocabulary/Definitions
Density: The measure of mass per unit volume, including the number and weight of molecules of a fluid per unit volume. p=m/V where m=mass and V=unit volume
Flow visualization: Techniques to make visible what happens inside of transparent fluids
Fluid: Any gas or liquid, including air and water.
Shear force: A force that acts sideways, along a surface (even an imaginary surface inside a fluid). This is at a right angle to pressure, which is a 'normal' force, pushing straight onto the surface.
Stratify: To form, arrange or deposit in strata or layers. To become layered.
Viscosity: A measure of how stiff a fluid is, or how easily, or quickly, it flows. The resistance of a fluid to shear forces. It is harder to slice honey with a knife than it is to slice water, thus honey has higher viscosity than water. Viscosity is a property of the fluid, like density or temperature.
Assessment
Pre-Activity Assessment
Discussion Questions: Solicit, integrate and summarize student responses.
- What would happen if we poured oil into a glass of water and why? (Answer: The oil should rise above the water, because it is less dense than the water.)
- What happens when you drop a coin into a water fountain? (Answer: The coin sinks to the bottom because the coin is denser than water.)
- What happens when you pour chocolate syrup into your milk and do not stir it correctly? (Answer: The syrup sinks to the bottom because syrup is denser than milk.)
Activity Embedded Assessment
Prediction: Have students predict what the fluids will do when they are all poured into one glass container. (Possible answers from students: They all will mix; they will separate in the order from bottom to top: corn syrup, strawberry syrup, shampoo, oil, alcohol.)
Worksheet: Have students complete the Density Rainbow and the Great Viscosity Race Worksheet; review their answers to gauge their mastery of the subject.
Post-Activity Assessment
What's in My Refrigerator? Ask students to test fluids from their refrigerator at home, and make an ordered list with the densest at the bottom of the list. Next, make a similar list for viscosity by racing different fluids against each other, and put the most viscous at the bottom of the list. How often is the denser fluid also more viscous? (Answer: Most of the time.)
Safety Issues
- Warn students that the food coloring can stain their clothes and fingers
- Warn students that alcohol stings if comes in contact with cuts or eyes.
- Remind students that the shampoo and isopropyl alcohol are not for drinking.
- In general, foods and hair/skin products can be safely mixed together, but household cleaners (cleaners for glass, laundry, floor, etc.) should not be mixed with anything.
- Materials from the rainbow and race can be disposed of down the sink.
Troubleshooting Tips
Make sure the ketchup and chocolate syrup are both at room temperature for the race to work right.
Alternate rainbow idea: Teachers could also provide each student with a clear plastic cup to make their own density rainbow.
Alternate rainbow idea: To save on materials, use a 100-150 ml glass beaker instead of the glass container or vase. The density column can still be observed in the beaker and requires only half the materials.
Activity Extensions
Have students guess the relative viscosity of the fluids used in the density rainbow activity, and then conduct viscosity races to test their hypotheses.
Have students race mixtures of the fluids from the density rainbow. Does mixing a fluid with another affect its viscosity?
Have the students brainstorm a list of machines and objects in their everyday lives that use or contain fluids. How are these items designed differently depending on which fluid it uses and/or contains? Remind students that fluids can be any gas or liquid, including air and water. Then, have the student write a one-page paper on what they observed. (Answers will vary. Ideas include: How the pipes are set up at their house to reach the shower and the water taps. How drains are positioned on our streets to drain and collect rainwater. How this explains the shapes of different bottles, such as water bottles being ridged, ketchup bottles being very flat on the sides, hair conditioner bottles many times have the opening on the bottom and tend to be cone-shaped [larger at the top than at the bottom]. There are many good examples relating to weather or floatation. Different densities may be observed on a lake, when the small amount of oil released by a motor boat rises to the surface of the lake, sometimes creating a rainbow on the water surface. An advanced observation might be how helicopter blades look similar to the boat propeller blades, yet they are different because helicopters work in air, while boat propellers work in water.)
Consider using this activity to relate the earth science concept of the layers of the atmosphere.
Conduct the Floating and Falling Flows (or demonstration) to learn more about the physics and beauty of fluid dynamics and buoyancy.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

tudents are introduced to the similarities and differences in the behaviors of elastic solids and viscous fluids. In addition, fluid material properties such as viscosity are introduced, along with the methods that engineers use to determine those physical properties.

Students discover fluid dynamics related to buoyancy through experimentation and optional photography. Using one set of fluids, they make light fluids rise through denser fluids. Using another set, they make dense fluids sink through a lighter fluid. In both cases, they see and record beautiful flui...

Students are introduced to the important concept of density. Students devise methods to determine the densities of solid objects, including the method of water displacement to determine volumes of irregularly-shaped objects.

Students explore an important role of environmental engineers—cleaning the environment. They learn details about the Exxon Valdez oil spill, which was one of the most publicized and studied human-caused environmental tragedies in history.
Copyright
© 2006 by Regents of the University of Colorado.Contributors
Cody Taylor; Gala Camacho; Jean Hertzberg; Malinda Schaefer Zarske; Denise CarlsonSupporting Program
Flow Visualization Laboratory, Department of Mechanical Engineering, College of Engineering and Applied Science, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: February 25, 2020






User Comments & Tips