Quick Look
Grade Level: 7 (6-9)
Time Required: 45 minutes
Expendable Cost/Group: US $6.00
Group Size: 3
Activity Dependency: None
Subject Areas: Physical Science
Summary
Students discover fluid dynamics related to buoyancy through experimentation and optional photography. Using one set of fluids, they make light fluids rise through denser fluids. Using another set, they make dense fluids sink through a lighter fluid. In both cases, they see and record beautiful fluid motion. Activities are also suitable as class demonstrations. The natural beauty of fluid flow opens the door to seeing the beauty of physics in general.
Engineering Connection
Engineers use their knowledge of fluids to invent, repair, reinforce and improve previous inventions that use liquids and gases. When engineers understand fluids, they can help make airplanes, ships, fire truck pumps, fans, water heaters, paper mills, glass factories and soda pop bottling plants. Even understanding the behavior of fluid flow from a cough or sneeze can help engineers design better tissues.
Learning Objectives
After this activity, students should be able to:
- Describe some aspects of buoyancy-driven flows.
- Understand that gravity, shear force and surface tension can affect fluid flow.
- See that visualizing a fluid flow can both show the physics that are occurring, and be beautiful.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Develop and design a scientific investigation to separate the components of a mixture
(Grade
7)
More Details
Do you agree with this alignment?
-
Predict and evaluate the movement of an object by examining the forces applied to it
(Grade
8)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 2 sheets of white paper (to use as a backdrop)
- Tape (to affix white paper securely to wall and tabletop)
- Pencils (for completing the worksheets)
- Student Instructions Handout, one per group
- Positive Buoyancy Worksheet, one per person
- Negative Buoyancy Worksheet, one per person
- Paper towels (in case of spills)
- Digital camera (optional)
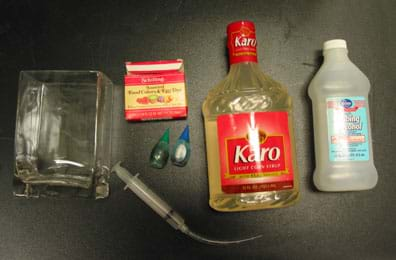
For Positive Buoyancy Station (see Figure 2):
- 1 rectangular glass container or vase (no smaller than 15 cm x 10 cm x 8 cm, or 6 in x 4 in x 3 in; available at hobby or craft supply stores)
- 32 oz (960 ml) corn syrup
- 16 oz (480 ml) isopropyl alcohol (99% recommended but 70% may be used)
- Water (exact amount depends on size of glass container)
- 1 syringe with needle removed (around 10cc; available at pharmacy or hardware stores)
- 4 in (10 cm) of 1/16th-in (.16 cm) diameter tubing attached to opening of syringe (tubing available at pet store as fish tank air line tubing)
- 1 small squeeze bottle of food coloring (blue or green preferred, but red may be used)
- 1 paper or plastic cup
For Negative Buoyancy Station:
- 1 rectangular glass container (no smaller than 15 cm x 10 cm x 8 cm, or 6 in x 4 in x 3 in; available at hobby or craft supply stores)
- Water (exact amount depends on size of glass container)
- 1 small squeeze bottle of food coloring (blue or green preferred, but red may be used)
- 1 syringe with needle removed (around 10cc; available at pharmacy or hardware stores)
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_flow_activity1] to print or download.Pre-Req Knowledge
Some knowledge of forces is helpful. Also, a basic knowledge of viscosity deepens students' understanding; as provided in the associated activity, Density Rainbow and the Great Viscosity Race.
Introduction/Motivation

(Optional: During the activity introduction, show students the images provided in the attached Flow Visualization Images PowerPoint presentation.)
What is the difference between a solid and a liquid? One answer is that when you push sideways on top of a solid, it can resist you without changing its shape. (Demonstrate by sliding your hands along the surface of a desk). This is known as resisting a shear force. By contrast, when you push sideways on the top of a liquid, it must move. This is why liquid films are slippery. Inside a car engine, a liquid film of oil is used as a cushion between moving parts. Ice is slippery when there is a liquid film of water on it. When you ice skate the pressure of your skate melts the ice right underneath it, so it slides easily.
When you drop a dense solid object into water, it just falls to the bottom. The water cannot resist being pushed out of the way. But when you drop a dense (heavier) liquid into a less dense liquid, different things can happen. If the liquids do not mix, like water into oil, then the denser fluid forms a sphere, and sinks mostly in that shape (as shown in Figure 3). This is called negative buoyancy, when the denser fluid sinks. (Optional: Demonstrate negative buoyancy as a demo by dropping colored water into a glass of oil, or add it to the negative buoyancy portion of the activity. The reason that oil and water do not mix is actually kind of complicated, and we will not cover it here, but the result is that oil is hydrophobic; i.e., tends not to combine with water.)
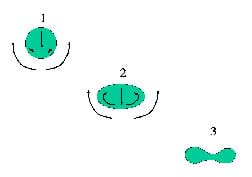
But if the liquids can mix, like food coloring or alcohol in water, then the shear forces (or forces acting sideways) between the two fluids can make beautiful shapes as the denser fluid sinks, or a lighter fluid rises (positive buoyancy). In these cases, the shear forces are created by viscosity(that is, friction) as gravity pulls the droplet downward. A droplet shape that starts out as a sphere gets deformed into a pill shape as the fluid in front of the droplet is pushed out of the way, and drags some of the droplet fluid with it. Next, the droplet is deformed into a shape like a blood cell (thinner in the middle), as shown in Figure 4.

Sometimes the fluid in the middle is pulled to the outer edge, leaving a vortex ring (as shown in Figure 5) such as a smoke ring. Eventually the ring of falling fluid may break up into smaller droplets, leaving a trail of partly mixed fluid behind it (as seen on the left side of Figure 1).
Similar things happen when a less dense fluid rises through a denser fluid. In Figure 6, dyed alcohol was injected into the bottom of the wine glass. It rises, first through corn syrup, and then through water. Notice the little vortex rings here and there. Another example is the right side of Figure 1, where a droplet of food coloring was dropped from above into sugar syrup. Its momentum carried it to the bottom, and then buoyancy made it rise.

In all of these images, the moving fluid was colored with a food coloring dye, and the surrounding fluid was left clear. This is a type of flow visualization, which is used to see what is going on in a fluid. Pure food coloring is slightly denser than water, but we will mix it with rubbing alcohol to make a mixture that is less dense. Flow visualization is important for understanding fluid flows for scientific and engineering purposes, such as the design of airplanes, water treatment processes, even soda pop bottling plants, but it is also beautiful to watch.
Fluids move any time a net (unbalanced) force acts on them. After a fluid is in motion, it has momentum, so more force is required to stop it, or change its direction. What are the forces here? Gravity is pulling downward, more strongly on the denser fluids (higher weight per volume), so lighter, less dense fluids are squeezed upward. If the fluids do not mix, surface tension pulls the droplets into spheres, and surface tension may hold some of the dye at the surface of the water. Friction from molecules bouncing off each other in the fluid results in viscosity, which then makes the shear force that deforms the droplets. As you go through these activities today, think about what forces are acting on the fluid and causing the motions you observe.
Procedure
- Divide the class into teams of three students each.
- Have half the teams start with the negative buoyancy experiment, and half with the positive, and then swap materials.
- This activity can also be performed as a class demonstration.
- Gather materials and make copies of the Positive Buoyancy Worksheet and Negative Buoyancy Worksheet, one each per person, and the Student Instructions Handout, one per group.
- Set up one buoyancy station per group, half with the negative buoyancy experiment and half with the positive, as described below.
Before the Activity
Assemble one buoyancy station per student group.
- It works best to set up the stations at locations with a white surface and a white background. Or, use a horizontal and vertical sheet of white paper, as shown in Figure 7.
- At each station, place 1 glass container, 1 paper cup, paper towels, 1 syringe, 2 small squeeze bottles of food coloring (blue, green), and 1 length of plastic tubing.
- Place the container on top of the white paper on the tabletop with its long side aligned with the long side of the paper on the tabletop.
- For the positive buoyancy stations, add 1 bottle of alcohol and 1 bottle of corn syrup.
- Distribute worksheets and instructions.
- Place a digital camera on each desk (optional).

Positive Buoyancy Station
- Pour into the glass container about 1.5 inches (3.8 cm) of corn syrup.
- Pour 2 inches (5 cm) of cool water on top of the corn syrup.
- Mix 3 drops of food coloring with about ¼ cup (59 ml) of isopropyl alcohol (blue food coloring recommended) in the cup.
- From the cup, pull the colored fluid up into syringe by submerging the tube tip and pulling back on the plunger.
- Push slightly to fill the tube until almost to the end.
- Insert the tube carefully into the lowest layer of fluid within the container, below the surface of the corn syrup, pointing the end of the tubing away from container walls (see Figure 8).
- Experiment with a small squirt of fluid first. What happens to the colored fluid in the corn syrup compared to what happens to it in the water layer? Make a sketch on the worksheet.
- Try a bigger squirt.
- Sketch and/or photograph the colored fluid, filling out the worksheet. If cameras are available, place the camera directly in front of the glass container on the white sheet of paper. Take close-ups, activating any close-up (macro) feature on the camera. Also take wider angle images that include the top waterline in the container.
- Complete the worksheet.
- Clean the station.

Negative Buoyancy Station
- Fill the glass container half full with warm water.
- Drip food coloring directly into the water, first one droplet, then several together. Observe what happens (see Figure 9).
- Experiment with dripping from different heights: ½ inch (1.3 cm), one inch (2.5 cm), 2 inches (5 cm), 4 inches (10 cm).
- Sketch and/or photograph the colored fluid, filling out the worksheet. If cameras are available, place the camera directly in front of the glass container on the white sheet of paper. Take close-ups, activating any close-up (macro) feature on the camera. Also take wider angle pictures that include the top waterline in the container.
- Complete the worksheet.
- Clean the station.

Vocabulary/Definitions
Buoyancy: The power of a fluid to exert an upward force on an object placed in it.
Flow visualization: Techniques to make visible what happens inside of transparent fluids.
Fluid: Any gas or liquid, including air and water.
Negative buoyancy: An object or parcel of fluid has negative buoyancy if it sinks.
Positive buoyancy: An object or parcel of fluid has positive buoyancy if it rises or floats.
Shear force: A force that acts sideways, along a surface (even an imaginary surface inside a fluid). This is at a right angle to pressure, which is a 'normal' force, pushing straight onto the surface.
Viscosity: A measure of how stiff a fluid is, or how easily, or quickly, it flows. The resistance of a fluid to shear forces. It is harder to slice honey with a knife than it is to slice water, thus honey has higher viscosity than water. Viscosity is a property of the fluid, like density or temperature.
Vortex ring: A torus (or donut) of spinning fluid. A smoke ring is an example of a vortex ring. The fluid spins from the middle of the ring (the hole) to the outside, and back again, not the long way around the ring.
Assessment
Pre-Activity Assessment
Brainstorming: In small groups, have students engage in open discussion. Remind them that no idea or suggestion is 'silly.' All ideas should be respectfully heard. Ask the students:
- What determines whether a solid object will float or sink in water?
- What determines whether another liquid will float or sink?
- What happens when a dense liquid falls through a lighter one? How is it different from what happens to a sinking solid object?
Activity Embedded Assessment
Worksheets: Have students record their observations on the Positive Buoyancy Worksheet and the Negative Buoyancy Worksheet. Review their answers to gauge their mastery of the subject. Have them discuss their answers among their groups.
Post-Activity Assessment
Discussion Questions: Solicit, integrate and summarize student responses.
- What are the forces that can make fluids move? (Answers: Shear, pressure, surface tension, gravity.) Give examples of these forces.
- List other flows that you have seen visualized. What forces were acting there? (Possible answers: Milk poured into water, chocolate syrup poured into milk, steam from a shower, oil film on a puddle, oil and vinegar mixed for salad dressing, smoke from a fire, steam plume from a power plant, exhaust from a bus or truck, blowing snow or rain, explosions on TV or movies, wind blowing over a lake.)
Show and Tell: Create a display of students' images of flow and hang it in the classroom, hallway or the library of your school. Have the students 'show and tell'to another class the flow visualizations they created, explaining their work to other students.
Homework
Fluids at the Movies: Many movies begin with images of fluid flow; often natural flows like clouds or waves at a beach. Keep a log of whether a movie starts with some sort of fluid flow for one week (or for the next 10 movies you watch). According to your statistics, what percentage of movies start this way?
Safety Issues
- Warn students that the food coloring can stain their clothes and fingers.
- Warn students that alcohol stings if comes in contact with cuts or eyes. It is also not for drinking.
Activity Extensions
Have students investigate other fluids in motion. Have them look for patterns of clouds outdoors or other examples listed in the Post-Activity Assessment discussion answer. Or, have them answer the question, what causes rain to fall? [Answer: The sun warms water, making water vapor, which mixes with warm air and rises. As it rises, it cools. When it cools enough, the vapor condenses to water again and therefore becomes denser than the air around it. In a cloud, air is moving upward fast enough to keep tiny water or ice droplets in the air, but if the droplets grow big enough they fall as rain or other precipitation.]
Conduct the Density Rainbow and The Great Viscosity Race activity to learn more about the densities and viscosities of fluids.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

tudents are introduced to the similarities and differences in the behaviors of elastic solids and viscous fluids. In addition, fluid material properties such as viscosity are introduced, along with the methods that engineers use to determine those physical properties.

Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics.

Students explore the densities and viscosities of fluids as they create a colorful 'rainbow' using household liquids. While letting the fluids in the rainbow settle, students conduct 'The Great Viscosity Race,' another short experiment that illustrates the difference between viscosity and density.
Copyright
© 2006 by Regents of the University of Colorado.Contributors
Cody Taylor; Gala Camacho; Jean Hertzberg; Malinda Schaefer Zarske; Denise CarlsonSupporting Program
Flow Visualization Laboratory, Department of Mechanical Engineering, College of Engineering and Applied Science, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: June 11, 2021






User Comments & Tips