Quick Look
Grade Level: 10 (9-12)
Time Required: 1 hours 45 minutes
(to conduct lesson and four activities over two class periods)
Lesson Dependency: None
Subject Areas: Chemistry, Physical Science, Science and Technology

Summary
Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. They also learn of the importance of surfactants, such as soaps, and their use in everyday life. Through associated activities, students explore how surfactant molecules are able to bring together two substances that typically do not mix, such as oil and water. This lesson and its associated activities are easily scalable for grades 3-12.Engineering Connection
Engineers use surfactants in many aspects of material development and industrial manufacturing. For example, surfactants are commonly used in paints and dyes to improve adhesive properties and ensure even spreading on building surfaces and consumer packaging. The concepts of molecular polarity and surface tension are widely researched and applied in many aspects of engineering science, including their end use in paints, dyes, cosmetics, lubricants, pharmaceuticals, and textile production. Surfactants can be found in almost every consumer product — from a Twinkie wrapper to the ink in a pen.
Learning Objectives
After this lesson, students should be able to:
- Define polarity and identify the differences between polar and non-polar molecules.
- Identify the impact of polarity on interactions between molecules (for example, oil and water, water on a surface).
- Describe surface tension and how it affects the shape of water.
- Calculate the surface area of a sphere and a cube and determine that a sphere has the least surface area for a given volume.
- Understand how surfactants act to reduce surface tension between polar and non-polar molecules and facilitate their mixing.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
DCI.PS1.A.9-12.1.
Each atom has a charged substructure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
DCI.PS1.A.9-12.3.
The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Interpret how good design improves the human condition.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Develop innovative products and systems that solve problems and extend capabilities based on individual or collective needs and wants.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Florida - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
Florida - Science
-
Recognize that atoms are tiny particles in materials, too small to see.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/usf_surfactants_les1] to print or download.Introduction/Motivation
Have you ever seen a duck in the water? A duck is able to maintain buoyancy on water not because it is less dense than the water but because its feathers are coated with oil that traps air and repels water. In order to swim under water, a duck exhales and pulls its wings against its body to squeeze out the trapped air. Upon resurfacing, it takes a deep breath and flaps its feathers to trap air again in the oily feathers.
A surfactant is a chemical that connects polar and non-polar molecules, allowing them to mix. In the duck example, introducing a surfactant would enable the water and the oil on the duck's feathers to mix. So, if a surfactant was added to the water of the duck's pond, it would disrupt the duck's ability to repel water and trap air bubbles, making it difficult or impossible for the duck to float. This is why when surfactants are used to clean up oil spills, the ducks have a hard time floating in the water.
So what exactly is a surfactant? Well, a surfactant is a molecule that lowers surface tension and allows for the mixing of dissimilar liquids. What is surface tension? (Listen to student definitions, then clarify if needed: Surface tension is a property of the surface of a liquid that causes an attractive or repulsive force between the liquid and another surface.) Surface tension is not always between two liquids. We also see surface tension between liquids and gases, and liquids and solids (such as the meniscus we see in a graduated cylinder).
What are dissimilar liquids? A common example of dissimilar (non-mixing) liquids is oil and water. When you have greasy dishes, does running water over them make the dishes clean? What if the water is hot? That's right! Running water over the dishes will not clean them; we have to use soap! Based on the definition I just gave you of surfactants, do you think soap could be a surfactant? Well let's see. Does it reduce surface tension? (Expect puzzled looks.) I'm not quite sure what that means either, so let's come back to it. Does it allow for the mixing of dissimilar liquids? Well, oil and water are not the same liquid and they don't usually mix unless soap is added. I think there is a good chance that soap is a surfactant! In fact, soap is one of the most common surfactants.
Can anyone think of other places where we might find surfactants? It may not be so intuitive, but surfactants are also used in paints, dyes, cosmetics, pharmaceuticals, textile production, lubricants and many other consumer products. As a matter of fact, if it weren't for surfactants, many products wouldn't exist or wouldn't work well.
Engineers, in particular chemical engineers, play a key role in finding the right type of surfactant molecules needed to produce or use in consumer products. Engineers want surfactants such as soap to be strong enough to wash the oil off dishes or your hands but not so strong that it damages your skin. Can you imagine what would happen if the wrong type of surfactant was used in your shampoo? If the surfactant were strong enough it could damage the skin cells on your head and cause your hair to fall out! Engineers make sure this doesn't happen by finding the best surfactant for the job.
(Proceed to share with students the content information provided in the Teacher Background section, to the depth suitable for your class. Then conduct the associated activities.)
Lesson Background and Concepts for Teachers
The Basics of Polarity
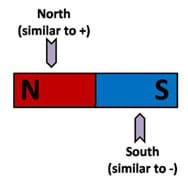
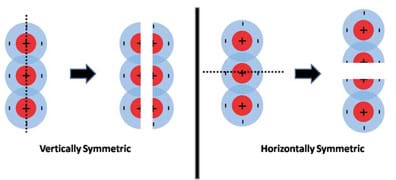
Magnets are simple macroscopic objects that can be used to demonstrate the general concepts of molecular polarity. The most fundamental concept behind polarity is the concept of magnetic attraction and repulsion. Every known magnet has two distinct regions or poles and each region or pole has a distinction. In the case of magnets, we refer to these poles as north and south. These terms originate from the early understanding that the Earth itself is a magnet and has a north and south pole. Figure 1 portrays a simple bar magnet and makes the connection between the concept of magnetic polarity and molecular polarity.
Figure 1 introduces the similarities between the concept of a magnet having a north and south pole and the basic charges of an atom: positive (protons) and negative (electrons). The main concepts of attraction and repulsion are outlined in Figure 2.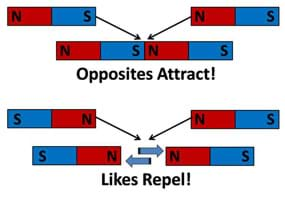
The general understanding of polarity in magnets can be extended to molecular polarity. It is important to have a firm understanding of the structure and nature of molecules and their charges. All molecules consist of electrons, protons, and neutrons. The most fundamental aspect of polarity arises from the spatial distribution (that is, the arrangement in the free space of the molecule) of the charged sub-atomic particles, the electrons and the neutrons. The structure of an atom is composed of a tightly packed nucleus containing the protons and neutrons around which electrons orbit. Because of this, most atoms have a shell of negative charges surrounding them such that, if the atom were probed at any angle, a negative charge would always be experienced before reaching the nucleus. If molecules, containing atoms with these electron shells, combine these shells equally, that is the electrons are equally distributed throughout, it can be said that these molecules have a uniform charge distribution. Molecules with a uniform charge distribution are generally considered to be non-polar in nature. The uniform distribution of charges is also referred to as a symmetric charge distribution and is a key concept in defining a non-polar molecule
Some molecules contain highly electronegative atoms, atoms that have a large affinity for electrons (for example, oxygen and nitrogen). In many of these molecules, the presence of the highly electronegative species results in non-uniformity in the distribution of electrons around the molecule as these electronegative atoms tend to have a greater attraction on neighboring electrons. This non-uniform distribution of electrons can give rise to regions of the molecule that have a more dominant positive charge (due to fewer electrons in that area) and other regions that have a more dominant negative charge (due to more electrons in that area). Molecules that have a non-uniform distribution of charges are also referred to as having an asymmetric distribution of charges, a key concept in defining a polar molecule.
Charge symmetry can be understood by looking at the atomic structure of a molecule. In Figure 3, the basic structure of a well-known polar molecule, water, is displayed. An accurate description and a student-friendly description are provided. Here we can see that the dominantly positive regions (presented in red) are located at one extreme while the dominantly negative regions (presented in blue) are at the other extreme. This is analogous to a bar magnet, which is also shown to reinforce this concept. In the case of the water molecule, if we were to poke at it with our finger we would feel two drastically different regions depending on which direction we decide to poke. On one end we would feel a lack of electrons, which is the positive pole of the molecule, while on the other end we would feel an abundance of electrons, which is the negative pole of the molecule. This is a fundamental concept behind the description of a polar molecule. 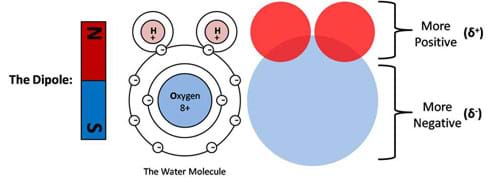
Another key aspect of polar molecules and water especially, is how the polarity of the molecules affects the overall order of a fluid. As we can see in Figure 4, water is highly ordered with each molecule attempting to align itself such that its positive pole is in close proximity to an adjacent molecule's negative pole. Just like magnets, polar molecules want to line up in a way that minimizes the repulsive forces caused by having two like poles in close proximity.
Furthermore, if we evaluate the charge symmetry of the water molecule, we can easily see that it is vertically symmetric but horizontally asymmetric (Figure 5). Since symmetry is defined as the property of obtaining a mirror image when an object is cut in half, the water molecule as a whole is considered to have an asymmetric charge distribution, a hallmark of all polar molecules.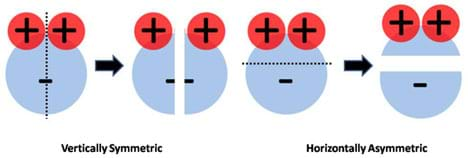
In contrast, an example of a non-polar molecule, a molecule with a symmetric charge distribution is carbon dioxide (CO2). Figure 6 shows a student-friendly (upper part) and a more accurate model (lower part) of the charge distribution in a CO2 molecule. As can be seen from these models, non-polar molecules such as CO2 have areas of positive charge on the inside whereas the outside is surrounded by negative charges. If we evaluate the charge symmetry of this molecule, as presented in Figure 7, it is apparent that regardless of how you cut the molecule in half, the two pieces will always be mirror images of one another, the hallmark of all non-polar molecules. Refer to the associated activity Get Your Charge Away from Me! to demonstrate the fundamental properties of polar and non-polar molecules (such as water and oil), how they interact and the affect surfactants (such as soap) have on their interactions. 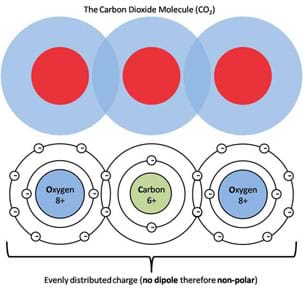
The Philia, the Phobia, and the Amphi of Molecules
Special concepts describe how different molecules and surfaces interact with water. The first concept is that of water-loving molecules and materials. These are referred to as hydrophilic. The word hydrophilic is derived from the Greek words hydros meaning "water" and philia meaning "friendship," literally translated to mean water-loving. Molecules that are hydrophilic tend to be polar in nature. The opposite of water loving is water-fearing and is referred to as hydrophobic, which originates from the Greek phobia meaning "fear." These molecules tend to be non-polar in nature.
Not all molecules are strictly polar and non-polar; some are large enough to contain both properties. These molecules are called amphiphilic molecules (see Figure 8). In order for a molecule to exhibit both properties, it must have a connecting chain. These chains are found in the form of polymer chains composed of a polar group at one end and a non-polar group at the other end, with a polymer chain filling in the span. These long chains contain molecules with symmetric charge distributions. The average distribution of charge along the chain is symmetric, making this region non-polar. At large enough distances, away from the polar "head group," other non-polar molecules can favorably interact with this chain ("tail group") without being repelled by the polar head group. This concept is further developed in the Surfactants section, below.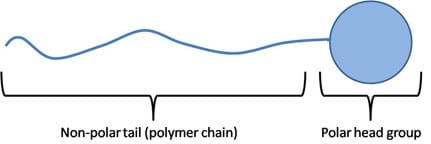
Oil and Water: Old Enemies
It is well known that oil and water do not mix and that oil is most often partitioned, separated, above water because it is less dense. But, what is happening that prevents oil and water from mixing like alcohol and water do? Oil and water do not mix because their molecular interactions are unfavorable; this involves polarity. Oils are non-polar substances that generally have an evenly distributed shell of electrons (negative charges) surrounding the molecules, whereas water is a polar substance that has distinct regions of both positive and negative charge. Considering the old scientific saying, "like dissolves like," polar molecules do not want to interact with non-polar molecules because of their dissimilarities in charge. Looking at the interface between oil and water as illustrated in Figure 9, it can be seen that water is very organized (as was shown before in Figure 4), aligning its positive poles to the adjacent molecules negative poles. For the purpose of this discussion and ease of illustration, the previously defined CO2 molecule is used to represent an oil molecule since both types of molecules have the same general charge distribution (in reality, oil molecules are much longer chains). Due to the lower density of oil, it partitions above the water without mixing. Additionally, the water molecules are ordered such that all of their positive regions are pointing towards the oil directly at the interface. This occurs because the oil is surrounded by negative charges and at the point where water and oil are "forced" to interact this is the most stable and favorable orientation. Another item worth noting is the complete lack of organization in the oil phase. Since no charge-based "motivation" exists to organize one way or another, they remain disorganized. This is due to the oil molecules being completely surrounded by negative charges.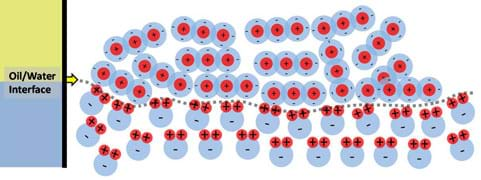
In real life, this could manifest as oil slicks after an oil spill. This is also exhibited by the lifting of oils on the road after a light rain shower. Engineers exploit this property to reclaim water from oil reservoirs that have been pumped dry. In reality, the reservoirs are not empty but the bulk liquid has been removed and the remnants are trapped in the surrounding sediment and rock. To extract this, they pump large quantities of water or a water/surfactant mix to drive out the oil from the rock and soil, and reclaim the remnants by other processes.
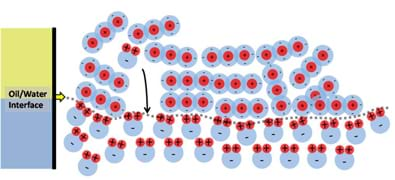
To illustrate how and why water and oil do not mix, let us take a single molecule of water and place it in the oil phase, as shown in Figure 10. The water molecule is completely surrounded by negative charges, which makes the positive pole of the water molecule "happy," but the negative pole does not like being so close to all of the surrounding negative charges. This situation causes the water molecule to flip around and move, much like when you try to bring the north poles of two magnets together. This motion pushes around surrounding oil molecules enabling the water molecule to slowly drop through the oil (due to being denser than the surrounding oil) and eventually find the oil/water interface as shown in Figure 11. When the water molecule reaches the water phase it can then happily align itself with the rest of the water.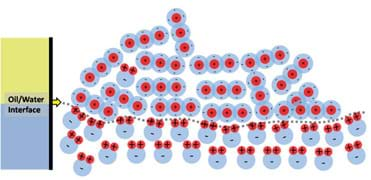
Surface Tension and Water
When water molecules rest on surfaces for which they do not have an affinity, they tend to dome up spherically off the surface. This is often due to the non-polar nature of the surface and the water's great dislike for interacting with it. In botany, this effect has been coined the Lotus effect; after the lotus plant that exhibits this trait. It is also present in many other waxy-leaved plants and a variety of fungi. Outside of nature, this same effect can be seen on the hood of freshly waxed car, on the outside of a window after a rain shower, or on other metal or treated wood surfaces. In these instances, the water molecules are being repelled from the surface. The reason for these phenomena follows the same logic presented above. Water does not like electron-rich, non-polar surfaces. (Following this logic, it is also true that water is affected by non-polar gases, but for this section we will focus on its interactions with solids.)
Water's disfavor of non-polar surfaces is expressed through an increase in surface tension and the formation of hemispherical shapes, which approach perfect spheres as the surface becomes increasingly non-polar. Surface tension is a property of the surface of a liquid that describes the cohesion of molecules to like molecules. The more non-polar a surface is, the stronger the cohesive forces between neighboring water molecules and thus, the higher the surface tension. Optional: For younger students, they can see surface tension in action with the fun and activity Down with the Clip! This principle is illustrated in Figure 12. When the surface is highly non-polar the water molecule is nearly perfectly spherical, when the surface is moderately non-polar the water molecule is more hemi-spherical, and when the surface is weakly non-polar the water molecule tends to spread across the surface. We note that surface tension angles (in Figure 12) between solids, liquids, and gases depend on the relative properties of each material considered.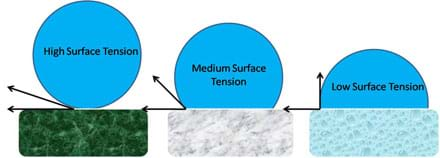
This behavior is also seen in other instances. When water precipitates, it falls in droplets. The stereotypical depiction of a rain drop is hemispherical with a conical tail. This is a misconception. If such droplets are viewed with a high speed camera, it can be seen that water forms perfect spheres. This is in part because it is more favorable for the water to interact with other molecules of its own kind than with that of the surrounding air. This is an example of the property of cohesion (attraction between similar or identical molecules.)
Water takes on a highly spherical shape when it interacts with non-polar surfaces because a sphere has the lowest surface area of any three-dimensional geometric shape. This is proven mathematically in the example below. It is favorable to have a low surface area when a large difference in surface energies (polar vs. non-polar) exists. This is analogous to crossing a hot street in a bathing suit. Given the task, a person would not cross the street on his/her hands and knees; rather, s/he would run across as fast as possible touching as little of the street as possible. Optional: For younger students, refer to the Tension Racers! activity for students to explore how water interacts with different surface types due to surface tension by racing water droplets down various surfaces and observing which exhibit the highest level of surface tension and move fastest.
Surface Area Proof
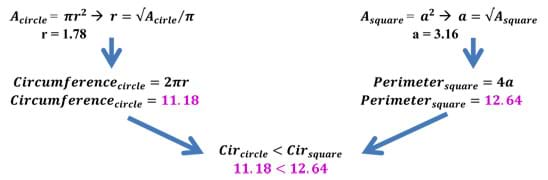
Example: Take a sphere and a cube of the same volume of 10 units. After doing some quick back calculation using equations for volume, we find the following radius and side length. Once the radius and side length have been calculated, we can use these lengths to determine the amount of surface area a specific shape (in this case, a sphere and a cube) have for the same volume (see Figure 13). As we can see, the surface area of the sphere is less than that of a cube of the same volume. In fact, the cube has almost 30% more surface area than the sphere! This example works with 2D figures as well. The circle has the least surface area of all shapes.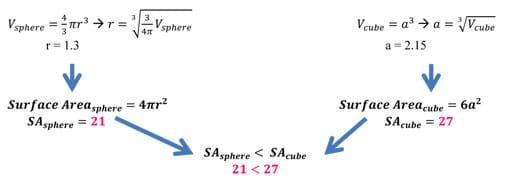
Surfactants
Because of the chemistry-borne polarity, hydrophobic and hydrophilic molecules do not energetically favor mixing (they are too "lazy" to mix). In order to make mixing favorable, a molecular intermediate is needed. This intermediate carries molecular "appendages" that are both polar and non-polar, and therefore are favorable to both groups, as illustrated in Figure 14. These intermediate molecules are commonly referred to as surfactants. Optional: For younger students, they can learn more about the importance and application of surfactants with the activity Oil and Water: Washing Up with Surfactants.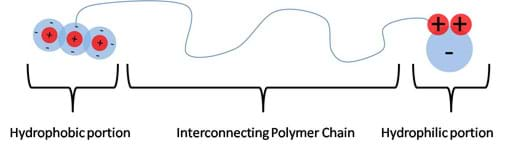
How do surfactants bring oil and water together?

A surfactant is generally an amphiphilic molecule capable of interacting with various types of molecules including polar and non-polar molecules. The general challenge to washing greasy dishes or dirty hands is that the dirt is usually encased in oil or grease. We usually use water to accomplish this task, but since oil and water do not mix well, the water generally beads on the dirty greasy surface and rolls away leaving behind the dirt. The key component in most soaps and detergents is the surfactant. Since these molecules are amphiphilic, they are capable of binding to both water and oil molecules, resulting in the liberation of the dirt. By binding to both water and oil, surfactants allow these molecules to mix (via reducing the surface tension between water and oil). To visually reinforce the concepts that pertain to surfactants, we present a student-friendly "superhero," surfactant man, in Figure 15.
What makes surfactant man such a special type of molecule is his amphiphilic nature. He doesn't mind grabbing on to either a polar molecule such as water or a non-polar molecule such as oil. Figure 16 is a generalization of this concept involving surfactant man. Here, he is capable of "holding" on to many non-polar and polar molecules all at once. 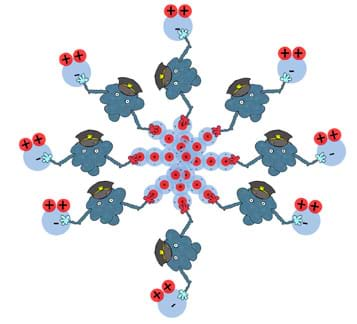
In the end, you have molecules that generally do not like to mix, in close contact with one another (see Figure 17 or the attached Surfactant Man Visual Aid).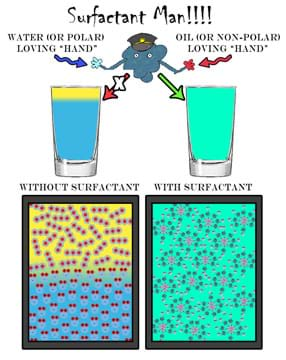
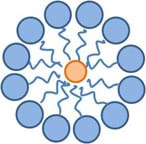
Please keep in mind that this is a generalization and the true nature of many surfactants involves the formation of spheres called micelles. A micelle is organized such that the hydrophilic heads of the surfactant molecule are on its surface while the hydrophobic tails of the surfactant molecule point towards its center. On the inside of the spherical micelle, components that interact with the hydrophobic tails (such as oil) can be trapped while the hydrophilic heads keep the entire sphere suspended in water. Micelles are not large in size and generally are composed of only a few dozen molecules or less. Figure 18 depicts the general concept behind micelles. This type of molecular organization can also be found in the cell walls of plants and the cell membrane of animal cells.
Associated Activities
- Get Your Charge Away from Me! - Students observe how water drops fall through oil and how the addition of surfactants drastically alters the shape of the falling water and enables the mixing of oil and water. This activity demonstrates the fundamental properties of polar and non-polar molecules (such as water and oil), how they interact and the affect surfactants (such as soap) have on their interactions. The activity is scalable for grades 3-12.
Vocabulary/Definitions
cohesion: Attraction between similar or identical molecules.
hydrophilic: A characteristic of having a strong affinity for polar molecules.
hydrophobic: A characteristic of having a strong affinity for non-polar molecules.
interface: A boundary between two systems or phases of matter.
lotus effect: The high water repellency exhibited by the leaves of the lotus flower. More broadly refers to the water-repelling properties of waxy-leaved plants, or high surface area to volume surfaces (nano-surfaces).
mixing: The ability for two fluids to be evenly dispersed in one another.
non-polar: Having globally equal charge distribution, resulting in charge symmetry.
polar: Having globally unequal charge distribution, resulting in magnetic (charged) poles.
repel: Resistant to something or incapable of mixing with it.
surface tension: A property of the surface of a liquid that causes an attractive or repulsive force between the liquid and another surface.
surfactant: A compound that reduces the surface tension between two dissimilar materials. Usually fluid-fluid or fluid-solid.
Assessment
Pre-Lesson Assessment
Discussion Questions: Solicit, integrate and summarize student responses. Ask the students:
- How does soap remove dirt and germs from your hands? (Have students offer suggestions to share with the class. Answer: Dirt and germs primarily accumulate in the oils on your skin. Soaps are made of surfactant molecules that bind together oils and water. This allows soaps to wash the dirt and germs off of your skin. Some soaps also have other additives with antiseptic properties.)
- What is a surfactant and where can we find them? (Answer: A surfactant is a molecule that has both polar and non-polar properties. Surfactants are used to lower the surface tension between two opposing fluids.)
Post-Introduction Assessment
Create a Surfactant: Have students create drawings that depict the idea of a surfactant. Require that drawings illustrate the function of this molecule and an architecture that they believe will enable the surfactant's functions. Next, have students propose different additives for their soaps (for example, antiseptics, perfumes or moisturizers). After this, have students describe how they might manufacture this soap if they were the head design engineers.
Lesson Summary Assessment
Surfactant Jeopardy: Divide the class into three or four groups. Ask each team to choose a name — something from the vocabulary of this lesson or after a surfactant-containing product. Have teams select a leader, who is in charge of the buzzer (either a real buzzer, bell or some other type of signaling sign). The teacher takes the role of game show host Alex Trebek and asks questions about the material that they have created and assembled in a Jeopardy-like fashion. Simulate the Jeopardy game by using note cards with point values and questions, or use one of the many freeware Jeopardy programs available on the internet.
Example questions/answers:
Q: The cause of molecular polarity. (Answer: What is charge distribution?)
Q: Molecules with this type of charge distribution are considered to be non-polar in nature. (Answer: What is a symmetric charge distribution?)
Q: A type of atom that has a high affinity for electrons. (Answer: What is an electronegative atom?)
Q: Polar molecules have this type of symmetry. (Answer: What is asymmetry?)
Q: A property responsible for the native orientation of water molecules. (Answer: What is the property of polarity?)
Q: A type of molecule that is said to "love water." (Answer: What is a hydrophilic molecule?)
Q: A type of molecule that bonds with both polar and non-polar molecules. (Answer: What is a surfactant molecule?)
Q: The link that connects together the polar and non-polar groups of a surfactant molecule. (Answer: What is a polymer chain? or What is a hydrocarbon chain?)
Q: The property of a liquid that is responsible for its cohesive properties. (Answer: What is surface tension?)
Q: A 3-D shape with the lowest surface area. (Answer: What is a sphere?)
Homework
Find Surfactant in Society: Have students develop a list of products or things that use or are composed of surfactants. Chemical engineers design these products to have specific properties for specific purposes. Examples: Dyes, inks, laundry detergents, cellular organisms, paints, skincare products, toothpaste, shampoos, pharmaceuticals, printing technologies, hard drives, solar panels, fire-fighting foam, ski and snowboard waxes, medical [for lungs], oil well extraction.)
Bonus Questions: Ask students to write answers in their lab books to the following questions: Would surfactants have the same characteristic properties if they did not have a long connecting polymer chain? What properties would they have?
Additional Multimedia Support
For fun, show students a 3-minute video of surface tension at work in zero gravity, made by NASA astronaut Don Petitt on the International Space Station. He uses candy corn to represent soap molecules to show how surfactant molecules work to clean grease or oil. See the Every Day is Science Friday's "Candy Corn in Space" video at -https://www.sciencefriday.com/videos/candy-corn-in-space-2/.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

This activity is an easy way to demonstrate the fundamental properties of polar and non-polar molecules (such as water and oil), how they interact, and the affect surfactants (such as soap) have on their interactions. Students see the behavior of oil and water when placed together, and the importanc...

Students experience firsthand one of the most common water treatment types in the industry today, flocculants. They learn how the amount of suspended solids in water is measured using the basic properties of matter and light. In addition, they learn about the types of solids that can be found in wat...
References
"Surfactants: Detergent Chemistry." n.d. Kiwi Web, Surfactants: Surface Active Agents, Chemistry and New Zealand. n.p., Accessed April 15, 2010. http://www.chemistry.co.nz/surfactants.htm
Copyright
© 2011 by Regents of the University of Colorado; original © 2011 College of Engineering, University of South FloridaContributors
Samuel DuPont; Ryan CatesSupporting Program
STARS GK-12 Program, College of Engineering, University of South FloridaAcknowledgements
This curriculum was developed by the USF Students, Teachers and Resources in Sciences (STARS) Program under National Science Foundation grant numbers DGE 0139348 and DGE 0638709. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: July 17, 2023




User Comments & Tips