Quick Look
Grade Level: 6 (5-7)
Time Required: 30 minutes
Expendable Cost/Group: US $0.50
Group Size: 5
Activity Dependency: None
NGSS Performance Expectations:

| MS-PS1-1 |
Summary
Students see how surface tension can enable light objects (paper clips, peppercorns) to float on an island of oil in water, and subsequently sink when the surface tension of the oil/water interface is reduced by the addition of a surfactant; such as ordinary dish soap.
Engineering Connection
The key property of a surfactant is its ability to reduce surface tension between two different molecule types, allowing for mixing when the molecules would otherwise want to remain separate. This property is extensively used in research and industry. For example, in the cleanup of the 2010 Gulf of Mexico oil spill, surfactants were used as dispersants. By spraying surfactants over the oil, the oil more thoroughly mixes with the surrounding water, spreading the oil over a greater area. This allows for more interaction between the oil and native microorganism that can digest the oil into less hazardous byproducts.
Learning Objectives
After this activity, students should be able to:
- Explain how surface tension allows objects that would normally sink to remain suspended on top of a liquid.
- Describe how a surfactant, such as soap, reduces the surface tension causing the floating object to sink.
- Define the molecular property that is responsible for surface tension.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to predict and/or describe phenomena. Alignment agreement: | Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. Alignment agreement: Solids may be formed from molecules, or they may be extended structures with repeating subunitsAlignment agreement: | Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Florida - Science
-
Demonstrate and explain that mixtures of solids can be separated based on observable properties of their parts such as particle size, shape, color, and magnetic attraction.
(Grade
5)
More Details
Do you agree with this alignment?
-
Identify the basic purpose of an experiment.
(Grade
5)
More Details
Do you agree with this alignment?
-
Recognize observable changes in a simple experiment, such as plant growth.
(Grade
7)
More Details
Do you agree with this alignment?
Materials List
For the teacher to use in the introduction:
- waxy-leaved plants, such as kale, cabbage or broccoli
- water
- soap (hand or dish)
Each group needs:
- 5 mL (1 tsp) oil (vegetable or olive)
- 250 mL (8.5 fl oz) of water
- 1 paper clip (or some large-ground peppercorns, which are easier to use with better reproducibility)
- 1 clear 12 fl oz (355 mL) cup
- soap (hand or dish)
Pre-Req Knowledge
A familiarity with the concepts of surface tension and the role a surfactant plays in lowering the surface tension between two dissimilar liquids. An understanding that surface tension in liquid systems is the result of the relative polarities of the liquids present.
Introduction/Motivation
(Have ready the plant leaves, water and soap to show students.)
When two liquids experience a high surface tension, they rearrange their molecules so that they limit contact with opposing molecules. Let's look at it this way: imagine you are walking across a very hot road in the summer time. How do you cross this road? Well you do so very quickly and on the least tender part of your foot; likely the ball of your foot. From this example it is easy to see why you wouldn't want to get down on your hands and knees to cross the road; you would burn a lot of skin! In a sense, this is what happens when polar and nonpolar fluids are mixed. They both do all they can to rearrange and avoid contact with one another. This effect is present in nature and is used by plants as a self-cleaning technique that we call the Lotus effect. When water contacts a plant that exhibits the Lotus effect, the water forms into nearly perfect spheres and rolls off the plant surface, picking up and removing any loose dirt or bugs. Here I have some [kale, cabbage, broccoli, etc.] to show you this effect. (Dribble water over the leaves.) Like oil and water, the leaf's surface and the water repel one another. This is due to the fact that the leaf is covered with waxy, oil-based, coating.
Polar and non-polar molecules repel one another — like the same poles of two magnets. Here, the oil or waxy coating is made from molecules that are primarily non-polar and the water is composed of molecules that are polar. Because of the properties of these molecules, we have strong repulsion, or surface tension. We also learned that a class of molecules called surfactants is capable of reducing the tension between opposing fluids. I have shown you the Lotus effect, now I will show you how the surface tension of the plant's leaf and the water is changed when soap is added to the water. (Add a few drops of liquid soap to the water.) Notice that the water no longer beads off of the leaf, but rather puddles on or "wets" the surface.
From past experience, most of us are aware that oil and water do not mix and that the oil stays separated and floats atop the water. It is important to mention that oil does not "bead" up on top of the water, but forms long thin islands. If you put a few drops of oil very far away from each other in a large tub of water they form little islands that eventually meet each other to form an even larger oil island floating on top of the water's surface. These islands form because of the surface tension between two fluids that do not like to mix (such as oil and water). Today, we are going to examine this system, the role surface tension plays, and how a surfactant affects the surface tension.
If we place a paper clip (or coarsely ground peppercorns) in water what, will happen? (Answer: It sinks.) However if we place it in oil, it tends to sit atop the oil because of its relative surface tension. In this activity, we are going to float a paper clip (or peppercorns) on the oil island floating atop the water and see how this is affected if the surface tension is lowered with the addition of a surfactant, soap.
Procedure
Background
The primary focus of this experiment is to illustrate the concepts of surface tension and visually demonstrate its effects in the case of an oil and water interface and how a surfactant can change these forces.
It is essential that each vocabulary word is briefly described in relation to the experiment so that efficient understanding of the experiment purpose is transferred.
Before the Activity
- Gather materials.
- Divide the class into teams of five students each.
- Make sure you have described to students the differences between polar and non-polar molecules and how this relates to mixing or dissolving. Additionally, a brief review of surfactants and a general picture of how they allow dissimilar fluids such as oil and water to mix can be beneficial.
- Review with students the vocabulary words and definitions.
With the Students
- Review with students the vocabulary words and definitions.
- Conduct the pre-activity assessment discussion, as described in the Assessment section.
- Fill a 12 oz. plastic cup ¾ full with water.
- Carefully add a small amount (1-2 tsp) of oil to the middle of the cup, forming an island of oil.
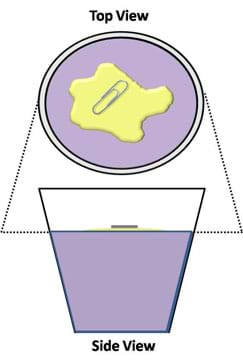
- Gently place a small paper clip (or some peppercorns) on the island of oil, such that they are suspend in the oil layer and not in contact with the water (see Figure 1).
- If using peppercorns, have students observe them from the side of the cup. Note that the pile of peppercorns creates a bowl in the surface by pushing the oil down into the water. Also, note that even though these are pushing into the water, the surface of the oil island does not break, thus preventing the peppercorns from falling into the water.
- Ask students to predict what will happen if a surfactant such as soap is added, as described in the embedded assessment in the Assessment section.
- Add a small portion (1-2 tsp) of dish soap near the edge of the cup, so not to physically disturb the surface tension.
- Have students pay close attention to changes in the surfaces on and around the oil island immediately after the soap is added.
- Observe and wait approximately 5 minutes for adequate reduction in surface tension, which causes the suspended item(s) to sink.
- Conclude with a class discussion. Did the soap act as a surfactant? How does this relate to the world around us? See the post-activity questions, answers and notes in the Assessment section.
Vocabulary/Definitions
lotus effect: The high water repellency exhibited by the leaves of the lotus flower. More broadly refers to the water-repelling properties of waxy-leaved plants, or high surface area to volume surfaces (nano-surfaces).
mixing: The ability for two fluids to be evenly dispersed in one another.
oil: A fluid (at room temperature) that is composed of non-polar molecules.
surface tension: A property of the surface of a liquid that causes an attractive or repulsive force between the liquid and another surface.
surfactant: A compound that reduces the surface tension between two dissimilar materials. Usually fluid-fluid or fluid-solid.
water: A fluid composed of polar molecules.
Assessment
Pre-Activity Assessment
What Floats on Oil?: Begin by generally discussing with the class what types of things can float on the surface of oil or water (separately). Establish that paper clips and peppercorns generally sink in water. This could also be extended to a discussion on buoyancy.
Activity Embedded Assessment
Make a Hypothesis: Have students observe the suspended paper clip (or a pile of peppercorns). Have them guess what will happen when a surfactant such as soap is added to the water. (Answer: The oil island will spread across the water surface and the paper clip/peppercorns begin to sink. In the peppercorn version, the peppercorns aggregate at the surface before falling to the bottom.)
Post-Activity Assessment
Did the Soap Act as a Surface Active Agent (Surfactant)?: Ask students why the surface acted the way that it did immediately after the soap was added. Also have the students discuss why the paper clip (or peppercorns) took some time to fall. Note, the paper clip may fall immediately, while the peppercorns take some time. (Answer: Time for mixing is required to lower the surface tension enough for the peppercorns to sink.)
How Does This Relate to the World Around Us?: Have students discuss why surfactants are important to our lives and the world around us. Some topics might include the washing of dirty hands and dishes. On a more global scale, a discussion concerning the use of surfactants in the cleanup of the 2010 Gulf of Mexico oil spill, as well as other chemical spills, can help to make a strong connection between the concepts learned in this activity and real-world engineering.
Troubleshooting Tips
If you have trouble floating a paper clip on the island of oil, try peppercorns instead. These are much easier to sprinkle on the island and a typical island can hold a generous amount of them.
If the oil island is difficult to observe, consider using oil-based food dye to improve contrast between the oil island and the water surface.
Activity Scaling
- For lower grades (grades 3-4), it may be more useful to conduct the activity as a visual demonstration in which students observe and discuss what is occurring.
- For upper grades (grades 5-7), have students conduct the activity in small groups and consider making this a competitive exercise in which the teams compete to find the largest (or heaviest) object that generally sinks in water that they can float on oil islands. Also, have each group define their controls and set a variable (volume of water, oil type and quantity, type of cup or mass of peppercorns) and run a series of experiments to see which variable has the greatest impact on the experiment.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students are introduced to superhydrophobic surfaces and the "lotus effect." Students learn how plants create and use superhydrophobic surfaces in nature and how engineers have created human-made products that mimic the properties of these natural surfaces.

Student teams are challenged to evaluate the design of several liquid soaps to answer the question, “Which soap is the best?” Through two simple teacher class demonstrations and the activity investigation, students learn about surface tension and how it is measured, the properties of surfactants (so...

Students culture cells in order to find out which type of surfactant (in this case, soap) is best at removing bacteria. Groups culture cells from unwashed hands and add regular bar soap, regular liquid soap, anti-bacterial soap, dishwasher soap, and hand sanitizer to the cultures.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 College of Engineering, University of South FloridaContributors
Samuel DuPont; Ryan CatesSupporting Program
STARS GK-12 Program, College of Engineering, University of South FloridaAcknowledgements
This curriculum was developed by the USF Students, Teachers and Resources in Sciences (STARS) Program under National Science Foundation grant numbers DGE 0139348 and DGE 0638709. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: April 22, 2022





User Comments & Tips