Quick Look
Grade Level: 12 (11-12)
Time Required: 2 hours 30 minutes
(Three 50-minute class periods)
Expendable Cost/Group: US $2.00
Group Size: 4
Activity Dependency: None
Subject Areas: Algebra, Measurement, Physical Science, Physics, Problem Solving, Reasoning and Proof
NGSS Performance Expectations:

| HS-ETS1-3 |

Summary
Student teams are challenged to evaluate the design of several liquid soaps to answer the question, “Which soap is the best?” Through two simple teacher class demonstrations and the activity investigation, students learn about surface tension and how it is measured, the properties of surfactants (soaps), and how surfactants change the surface properties of liquids. As they evaluate the engineering design of real-world products (different liquid dish washing soap brands), students see the range of design constraints such as cost, reliability, effectiveness and environmental impact. By investigating the critical micelle concentration of various soaps, students determine which requires less volume to be an effective cleaning agent, factors related to both the cost and environmental impact of the surfactant. By investigating the minimum surface tension of the soap, students determine which dissolves dirt and oil most effectively and thus cleans with the least effort. Students evaluate these competing criteria and make their own determination as to which of five liquid soaps make the “best” soap, giving their own evidence and scientific reasoning. They make the connection between gathered data and the real-world experience in using these liquid soaps.Engineering Connection
The study of surfactants, surface tension and the critical micelle concentration has many engineering applications. In the search for more efficient extraction of oil from underground reservoirs, primary and secondary techniques (pumping and washing with water) only remove ~30% of the total oil present. Using enhanced oil recovery techniques, chemical and petroleum engineers design surfactants that are low-cost, safe and effective at greatly reducing the surface tension because when the surface tension is lowered enough, trapped underground oil can be more easily washed out of the small pores of rock structures.
As another example, chemical engineers design soaps and cleaners to lower the surface tension of the water, which lowers the force between molecules, enabling water to more effectively bond with dirt and oil particles during washing, and thus achieve cleaner dishes and hands. Engineers in this field design soaps to be cost effective, good cleaning agents, non-toxic and efficient.
As an example of electrical applications, chemical and electrical engineers manipulate the surface tension of printer ink used in inkjet printers to specifically control the droplet size sprayed onto paper. Larger droplets require much larger surface tension to hold the droplets together. So engineers design ink that has low surface tension so that only small droplets can form, therefore enabling the creation of high-resolution images (high dots per inch, or dpi).
Learning Objectives
After this activity, students should be able to:
- Define surface tension and describe how it can be measured.
- Describe the effects of surfactants like soap on surface tension.
- Relate the surface tension of an aqueous solution to its ability to clean.
- Name some real-world applications in which the control of surface tension is important.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics, as well as possible social, cultural, and environmental impacts. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Evaluate a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | When evaluating solutions it is important to take into account a range of constraints including cost, safety, reliability and aesthetics and to consider social, cultural and environmental impacts. Alignment agreement: | New technologies can have deep impacts on society and the environment, including some that were not anticipated. Analysis of costs and benefits is a critical aspect of decisions about technology. Alignment agreement: |
Common Core State Standards - Math
-
Create equations in two or more variables to represent relationships between quantities; graph equations on coordinate axes with labels and scales.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Determine the best approach by evaluating the purpose of the design.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
design and implement investigative procedures, including making observations, asking well-defined questions, formulating testable hypotheses, identifying variables, selecting appropriate equipment and technology, and evaluating numerical answers for reasonableness;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
make measurements with accuracy and precision and record data using scientific notation and International System (SI) units;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
demonstrate basic principles of fluid dynamics, including hydrostatic pressure, density, salinity, and buoyancy;
(Grades
10 -
12)
More Details
Do you agree with this alignment?
-
demonstrate safe practices during laboratory and field investigations; and
(Grades
11 -
12)
More Details
Do you agree with this alignment?
Materials List
For the teacher’s class demonstration 1:
- 1 beaker of water
- 2 paper clips
- 1 small piece of toilet paper
- liquid hand dish soap, a few drops, right from the bottle
For the teacher’s class demonstration 2:
- 2 beakers
- water
- liquid food coloring
- liquid hand dish soap, 5 ml per 100 ml of water
- 2 small capillary tubes
Each group needs:
- set of 5 capillary tubes; thinner is better, such as a package of 500 0.8-mm capillary tubes (100 mm long x 1 mm OD; part # CT95-03 TLC spotting capillary tubes) for $30 from CTech Glass at https://www.ctechglass.com/ctech-08mm-id-glass-tlc-spotting-capillary-tubes-length-100mm-500pcspkg-p-376.html?gclid=CIDRtOeLoc4CFYKFaQodsckOwg
- ring stand base
- 2 ring stand rods
- 90° clip for rods
- 5 ml plastic syringes; no needles necessary, larger syringes may be used; such as from Vitality Medical at http://www.vitalitymedical.com/oral-medication-syringes-with-catheter-tip-by-monoject.html?gclid=CL6ylLeNoc4CFYU2gQodaUcOmA
- 1 glass 300-ml beaker
- ruler
- protractor
- camera (a phone is fine)
- safety goggles
- masking tape
- Surface Tension and Capillary Tubes Worksheet, one per student
- (optional) graph paper, computer with Excel® software or graphing calculator, to graph data
To share with the entire class:
- 5 liquid hand dish soaps of various brands; estimated ~10 ml per group (of a variety of brands) plus ~25 ml for class demo (brand does not matter)
- water
- paper towels

Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/rice_surfactants_activity1] to print or download.Pre-Req Knowledge
Students should be able to use a protractor to measure angles, a ruler to accurately measure distance, a graduated cylinder to accurately measure volume, their algebra skills to manipulate variables, and have a basic understanding of forces and static equilibrium.
Introduction/Motivation
(Introduce the concept of surface tension by conducting the following two class demonstrations.)
Demo 1
(For this demo, have ready a beaker of water, two paper clips, a small piece of toilet paper [somewhat bigger than a paper clip], and bottle of liquid hand dish soap. Hold the paper clip in your hand so that the class can see it.)
I hold in my hand a bare paper clip, made of steel wire. In a moment I will lay this paper clip on top of the water in this beaker. What do you expect to happen to the paper clip?
(Expected student predictions: It will sink; it will float. Ask students to justify their reasoning. For example, “The paper clip will sink because steel is denser than water and more dense objects sink in liquids.”)
Watch carefully as I drop the paper clip in. Those of you in the front, please share what you observe.
(Lay the paper clip on the water and watch it sink to the bottom. No need to be careful here.)
You are already familiar with buoyant force: the force that acts upward on an object due to a pressure difference on the top and bottom of that object. How did the buoyant force on this paper clip compare to the weight of the paper clip?
(Answer: The upward buoyant force on the paper clip from the water displaced was not enough to overcome the weight of the paper clip and therefore it sank. From the perspective of forces, a net force downward existed and the paper clip began to accelerate downward through the liquid. It may or may not continue to accelerate depending on resistive forces [drag] in the water.)
(Now hold up a second paper clip and a piece of toilet paper.)
Now I am going to change the scenario. I will place the new paper clip on a single sheet of toilet paper and gently float it on the surface of the water. Watch how this behaves differently.
(Make sure the paper is not so large as to stick to the sides of the container. After a short time, expect the paper to become soaked and sink to the bottom. Without disturbances, the paper clip is left floating on top of the water surface.)
Does anyone have an explanation for why the paper clip now floats? Has the density of water or the paper clip changed? Try to explain what you see in terms of forces.
(You are trying to get students to realize that some new force must be present and acting on the paper clip, a force that did not previously exist. This force is called surface tension, and is related to how strongly the water molecules attract to one another.)
(Now hold up a bottle of hand dish soap. Now is also a good time to show the class Figure 1.)
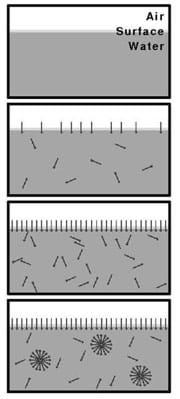
Chemical engineers have designed hand and dish soap to do several things to water. The soap is made of molecules called surfactants—surface active agents—that travel to the surface of a liquid. One part of this molecule is hydrophobic—water fearing—and one part of this molecule is hydrophilic—water loving. These molecules build up at the liquid-gas boundary so that the hydrophobic portions stick into the air away from the water molecules while the hydrophilic portions are still submerged. This concentration of surfactant on the surface greatly reduces the surface tension of the liquid. What will happen to the paper clip if soap is added to the beaker?
(Expected student predictions: It will sink; it will float for a time and then sink; it will still float. Again ask for physical reasoning and the use of scientific terminology. For example, “I expect that the paper clip will begin to sink because as soap is added, the surface tension decreases between the water and air; causing less upward force on the paper clip. And since the downward force of gravity on the paper clip has not changed, the paper clip will accelerate downward through the water.”)
Now watch this. (Add a few drops of the soap [right from the bottle] to the water with the floating paper clip.) What happened? (Listen to student explanations.) The added soap reduces the surface tension of the water and causes the paper clip to sink.
Demo 2
(For this demo, have ready two beakers, food coloring, two small capillary tubes and liquid hand dish soap. In advance, prepare two different solutions. In one beaker, place water with food coloring. In the other beaker, place water, soap and food coloring—a concentration of 5 ml of soap per 100 ml of water works great. Using a different color of food coloring for each solution helps to make the demonstration more visible to everyone in the class.)
I have two beakers on my desk. One contains colored water only. The other contains colored water with soap. Based on the last demonstration, how do you expect the surface tensions of these two solutions to compare?
(Expect students to say that the soap water probably has a lower surface tension.)
Does anyone have any ideas about how we might be able to measure this surface tension? How do we measure the force between these water molecules at the liquid-gas interface?
(Expect some interesting ideas from students; address the benefits and weaknesses of each. For instance, force scales can be used in lab settings to measure this, but they must be very sensitive. Spring scales will not work. Some students may suggest adding bigger and bigger paper clips until they sink—a good qualitative measure.)
I’ve heard some interesting ideas! I’m going to suggest an old method—the first method ever developed to measure surface tension somewhat reliably. I have in my hand two capillary tubes. They are simply thin glass pipes. Your previous chemistry experience will help you at this point. Why is measuring water in a graduated cylinder a little difficult? What happens to the water near the glass sides?
(Expect students to draw upon their hands-on experience in previous classes in which they learned about a meniscus and what it looks like for aqueous solutions. While students are usually taught to measure height from the bottom of a meniscus, they may not know why it happens.) Water bonds to the walls of the container and is pulled upward, even against the force of gravity. The stronger the surface tension, the stronger these bonds, and the higher the water will rise. This is capillary action as seen in plants, paper towels and thin tubes like these! I am about to insert one tube into each solution, in which do you think water will rise the highest? (Listen to student predictions.)
(Insert two clean, dry, identical capillary tubes into the two solutions and wait for the level to rise inside the tubes. Note that the thinner the tube, the higher the water will move and thus be more visible.)
Notice that the pure water rises higher than the water with soap and therefore has a higher surface tension!
Procedure
Background
Water has a high surface tension. Surfactants like soap can be added to water to reduce surface tension. Reducing water’s surface tension makes it easier for the water to clean dishes, move oil and move printing inks. Surfactants reduce surface tension by migrating to an air-liquid interface. This happens because the hydrophilic (water loving) head of the surfactant is attracted to the water phase and the hydrophobic (water hating) tail of the surfactant is repulsed by the water phase and attracted to the air or oil phase. The water phase has greater attraction to the hydrophilic portion of the surfactant than to other water molecules and this leads to the reduction in surface tension.
The critical micelle concentration (CMC) is the point at which the hydrophobic tails of the surfactants become attracted to each other. The surfactants from into spheres with the hydrophilic heads on the outside touching water and the hydrophobic tails touching each other, creating a waterless pocket inside the micelle. Once the CMC is reached, all new surfactants form micelles instead of migrating to the phase interface.
One application of surfactants is in ink jet printing. Surfactants are used to lower the surface tension of ink droplets, which leads to the ability to form smaller ink droplets, which enables greater precision and control in printing applications.
Another application of surfactants is in oil recovery. Reducing the surface tension in an underground oil mixture makes it easier to draw the oil out of small pores and caverns. Doing this increases the amount of oil that can be withdrawn from wells, and thus reduces the energy cost necessary to harvest the oil out of the ground.
Pros of low-CMC soap: This type of soap requires the least amount in order to make water foamy and optimal for cleaning. Adding additional soap past the CMC does nothing to improve cleaning efficiency.
Pros of lowest minimum surface tension: This type of soap makes it the easiest to remove dirt, grime and debris. The lower the surface tension the less energy that is required to clean a dirty object.
Before the Activity
- Gather the materials for the activity. If the capillary tubes and syringes are not on hand, refer to the links and potential suppliers to acquire them.
- Make copies of the Surface Tension and Capillary Tubes Worksheet.
- Practice the toilet paper and paper clip demonstration before conducting it in front of the class in order to acquire the right touch to perform it smoothly.
- Since finding the critical micelle concentration (CMC) of a surfactant takes some time, it is best to give each group only one soap to test. So, to test and compare five different liquid soaps on Day 3, you will need at least five groups. If you have more than five groups, give some groups the same surfactant to test, which can also be helpful to compare test data for the same soap product.
With the Students—Day 1: Introduce Surface Tension and Surfactants
- Introduce the concept of surface tension by conducting the two class demonstrations described in the Introduction/Motivation section.
- Discuss with the class some everyday engineering applications of the scientific concept of surface tension including oil recovery, soap design, and ink jet printing. Lower surface tension means better dissolving of dirt molecules (which are typically oily), better cleaning, and smaller droplets for printing. Refer to the Engineering Connection and Background sections.
- Discuss with the class how surfactants migrate to the water’s surface, lower surface tension, and eventually form micelles once the critical micelle concentration (CMC) is reached. Beyond this concentration, the surface tension of the liquid will no longer drop.
- Explain to students the open-ended engineering team challenge of this activity: To analyze the design and properties of five soaps to determine which soap is the “best.” While you may use different criteria to determine what is “best”—you must support your arguments with scientific data and reasoning.
- Hand out the worksheet. Explain the worksheet and its homework assignment in which students derive an expression in variables for the surface tension inside of a capillary tube. Have students complete the derivation portion in class so that you can provide guidance and feedback before they move on to the mathematical practice problems that are also provided.
With the Students—Day 2: Find Surface Tension at or Beyond CMC
- Divide the class into groups of four students each.
- Give each group a small syringe full of liquid soap to investigate and determine the surface tension of the soapy water when it has reached (or gone beyond) the critical micelle concentration (CMC). To do this, students design an investigation to measure the surface tension using the height of the soapy water column above the surface of water inside a beaker.
- Guiding Questions:
- How do you intend to find surface tension? (Answer: Measure the height of the water column and use equations derived on the worksheet.)
- How can you be sure you have reached the critical micelle concentration (CMC)? (Answer: The height of the water column will stop increasing as more soap is added.)
- What units will your answer have? Why? (Answer: Newtons per meter, N/m. Surface tension is a force per unit length.)
- What checks might indicate that you have made a mistake? (Answer: An increase in surface tension as more surfactant is added. Compare the measured height of the water column to the calculated result.)
- Closure Questions:
- At the end of this day’s investigation, ask students to complete a short answer ticket before leaving. Ask the students: Based upon today’s data, which soap (surfactant) is the best? Why? What impact does this have on everyday use?
- If time permits, have some groups share their answers with the class. If two groups disagree, have them support their findings and compare data. Perhaps the testing methods need to be reevaluated? Or perhaps the groups arrived at similar data, but interpreted it differently?
With the Students—Day 3: Test to Find CMC and Surface Tension of Different Soaps
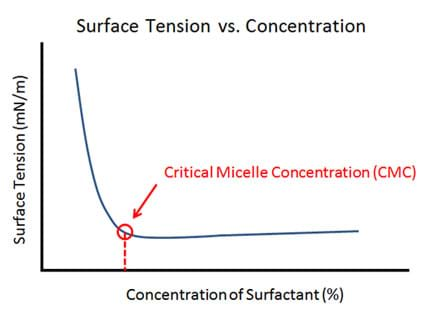
- Explain to the class: In this investigation, the class will test and compare five different liquid hand dish soaps of various brands. Each group will design a new lab to determine the critical micelle concentration (CMC) of the one soap it has been given. (If more than five groups, indicate that some groups will be testing the same soap, which will be a check on the test results.) This means that each group must start with water and slowly add a known amount of surfactant using a syringe, and calculate the surface tension each time based upon the height of the water column in the capillary tube. Eventually, when the surface tension no longer drops with the addition of soap, the CMC has been reached. (Show students the Figure 2 graph to help them visually represent this data.)
- Ask students to take measurements at each concentration and graph the data they obtain. The “corner” in the graphed line at which surface tension no longer falls denotes the CMC (see Figure 2).
- Tips: Concentration is easier to measure in terms of volume rather than mass. If adding 1 ml of soap to 300 ml of water, students can calculate that this as: 1/301 * 100 = 0.33% soap solution. The addition of another 1 ml of soap to the 301 ml of solution would yield a solution that is 2/302 * 100 = 0.66% soap solution. Between trials, rinse out the capillary tube with water and blow air through it to dry it.
- Guiding Questions:
- How will you find the CMC? (Answer: Find the “corner” on the plotted data.)
- How can you be sure you have reached the CMC? (Answer: Column height remains constant.)
- What units will your graph have? (Answer: N/m vs. concentration.)
- For soaps, what does a higher versus a lower CMC mean? (Answer: A lower CMC means less soap is needed for optimal cleaning power.
- Closure Task: Estimate for the class the critical micelle concentration of your assigned soap. It may help to graph your data on a computer, by hand, or with graphing calculator to make this determination. Share your data with the class so everyone can create final lab reports in which they decide which is the “best” soap.
- Conclude by assigning students to individually examine the class data and write a summary lab report, as described in the Assessment section.
Vocabulary/Definitions
capillary action: The ability of a liquid to move through narrow spaces due to attractive forces (adhesion) between molecules of the liquid and the solid walls that contain it. Also called capillary motion, or wicking.
critical micelle concentration: The minimum concentration of surfactants necessary for any added surfactant to form a micelle.
surface tension: The force per unit length at the air-liquid interface due to attraction between (cohesion) liquid molecules.
surfactant: A molecule with hydrophobic and hydrophilic portions that migrates to surfaces between phases of matter and lowers surface tension at that boundary.
Assessment
Pre-Activity Assessment
Predictions: Conduct a pre-activity assessment as part of discussions during demo 1 while presenting the Introduction/Motivation content. In particular, pay careful attention to students’ answers to the “predict” questions, which can give clues to their previous knowledge and misconceptions. If desired, have students record these predictions on paper before completing each step of the demonstration. Doing this can be an effective teaching tool because it forces students to confront their misconceptions since they are written in a tangible form.
Activity Embedded Assessment
Worksheet: Students complete a derivation relating the surface tension of a liquid and the height the liquid rises in a capillary tube, as guided by the Surface Tension and Capillary Tubes Worksheet. Mathematical practice problems of this relationship are also included. Review student answers to gauge their depth of comprehension.
Exit Questions: At the end of Day 2, ask students to complete a short answer ticket before leaving:
- Based upon today’s data, which soap (surfactant) is the best? Why?
- What impact does this have on everyday use?
Discussion Questions: Use the various guiding questions provided throughout the activity Procedure section to probe students’ understanding regarding what they are measuring, why they are measuring these values, and what that data can tell them. If students have trouble answering these questions in a coherent fashion, they may need more teacher guidance as they proceed.
Post-Activity Assessment
Lab Report: Assign students to each examine the class data and create a final lab report that answers the following questions:
- Of the five soaps investigated, which was the best surfactant? Support your claim using evidence obtained during your investigation. Make sure to describe the design criteria for your definition of “best.” Present your data neatly in charts, graphs and tables.
- According to your evidence, what does your data about this “best” soap mean when used at home to clean dishes?
- What would the average person experience with this soap that s/he may not experience with others?
- Does it have any disadvantages?
(Answer key: It is important to note that a “best” soap probably does not exist in every sense of the word. It is expected that one soap will have the lowest CMC, meaning that less raw material must be used to get the water “soapy” enough to clean efficiently. Thus, that particular soap will be less expensive and less wasteful for average consumers. In all likelihood, a different soap will have the lowest minimum surface tension, meaning that it will clean the most effectively and require less scrubbing by the user. It is up to the students to decide and explain, based on experience and data comparison between the surfactants, which one they each consider to be the “best.”)
Safety Issues
- Students should wear safety glasses while handling lab materials.
- Students should not eat or drink any of the lab materials.
- Since the lab involves soap (surfactants), hands and glassware can become slippery, so alert students to be cautious when moving capillary tubes and/or beakers.
- To remove residue, have students wash their hands at the end of the activity each day.
- Resulting soap and water mixtures are safe to pour down the drain.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students are presented with a short lesson on the difference between cohesive forces (the forces that hold water molecules together and create surface tension) and adhesive forces (the forces that causes water to "stick" to solid surfaces. Students are also introduced to examples of capillary action...

Students culture cells in order to find out which type of surfactant (in this case, soap) is best at removing bacteria. Groups culture cells from unwashed hands and add regular bar soap, regular liquid soap, anti-bacterial soap, dishwasher soap, and hand sanitizer to the cultures.

Students see how surface tension can enable light objects (paper clips, peppercorns) to float on an island of oil in water, and subsequently sink when the surface tension of the oil/water interface is reduced by the addition of a surfactant; such as ordinary dish soap.
References
Holmberg, Kister, Jönsson, Bo, Kronberg, Bengt, and Lindman, Björn. Surfactants and Polymers in Aqueous Solution (2nd edition). West Sussex, England: John Wiley & Sons, 2003. http://rushim.ru/books/polimers/surfactants-and-polymers-in-aqueous-solution.pdf
ShamsiJazeyi, Hadi, Miller, Clarence A., Wong, Michael S., Tour, James M., and Verduzco, Rafael, “Polymer-Coated Nanoparticles for Enhanced Oil Recovery.” Journal of Applied Polymer Science. (March 6, 2014) Vol. 131, 40576; doi: 10.1002/app.40576. http://onlinelibrary.wiley.com/doi/10.1002/app.40576/abstract
Copyright
© 2016 by Regents of the University of Colorado; original © 2015 Rice UniversityContributors
Shawn Richard; Lauchlin BlueSupporting Program
Nanotechnology RET, Department of Earth Science, School Science and Technology, Rice UniversityAcknowledgements
This material was developed in collaboration with the Rice University Office of STEM Engagement, based upon work supported by the National Science Foundation under grant no. EEC 1406885—the Nanotechnology Research Experience for Teachers at the Rice University School Science and Technology in Houston, TX. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or Rice University.
Last modified: May 4, 2017





User Comments & Tips