Quick Look
Grade Level: 6 (5-7)
Time Required: 30 minutes
Expendable Cost/Group: US $2.50
Group Size: 4
Activity Dependency: None
Summary
Students see how different levels of surface tension affect water's ability to move. Teams "race" water droplets down tracks made of different materials, making measurements, collecting data, making calculations, graphing results and comparing to their predictions and the properties of each surface, determining which surface exhibits the highest (or lowest) level of surface tension with water. They apply their results to make engineering recommendations for real-world applications.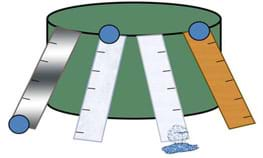
Engineering Connection
The material properties of surfaces are important in engineering design and innovation. Being able to create or treat surfaces on which water does not "stick" has been given much attention in engineering. For example, chemical engineers continually design more effective glass coatings that cause water to easily roll off, preventing a build-up of water on surfaces such as car windshields. Doing this requires an understanding of the concept of surface tension and how water behaves on different surfaces.
Learning Objectives
After this activity, students should be able to:
- Identify the differences in the interaction between water molecules and various surfaces (water drops form different shapes on different surfaces).
- Establish a connection between the surface tension of a surface and the shape a water drop forms on that surface and the speed with which the water moves across the surface.
- Identify that surface tension is a force and that it acts to pull water molecules together to form a sphere unless another surface pulls on or attracts the water molecules causing the sphere to deform.
- Identify that the amount of deformation of the water sphere is related to how much the water is attracted to or repelled by the surface.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Use models to describe phenomena.
(Grade 5)
More Details
Do you agree with this alignment?
-
Make observations and measurements to produce data to serve as the basis for evidence for an explanation of a phenomenon.
(Grade 5)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
-
Fluently divide multi-digit numbers using the standard algorithm.
(Grade
6)
More Details
Do you agree with this alignment?
-
Display numerical data in plots on a number line, including dot plots, histograms, and box plots.
(Grade
6)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Design problems are seldom presented in a clearly defined form.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Florida - Math
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
-
Fluently divide multi-digit numbers using the standard algorithm.
(Grade
6)
More Details
Do you agree with this alignment?
-
Display numerical data in plots on a number line, including dot plots, histograms, and box plots.
(Grade
6)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 1-2 ft (30-60 cm) strips each of four different materials for "runways," such as wood, metal, glass, plastic wrap, wax paper, etc. (let your imagination guide you)
- 1-2 ft (30-60 cm) strips of scrap cardboard or plastic, on which to wrap non-rigid racetrack materials
- something to elevate one end of the material strips (such as a shoebox or a wall)
- several sticks of modeling clay, to attach the strips to the elevated support
- several drops of water
- metric ruler
- pen or marker
- scissors
- tape
- stopwatch
- eye dropper (or more eyedroppers per team if you want to race drops simultaneously)
- (optional) paper towels, for wiping off tracks and for clean-up
- (optional) food coloring
Pre-Req Knowledge
A familiarity with stopwatches and their operation. Ability to record data in chart format.
Introduction/Motivation
(Have handy some chalk to make a few drawings and notations on the classroom board.)
Have you ever wondered why a water drop slides faster on some things than others? Sometimes, if you look closely, you can see a water drop hanging on the edge of a leaf or a piece of wood. What is holding that water drop up that prevents it from falling?
Everyone has used a paper towel before, right? When you put the edge of a paper towel into water what happens? It appears that the water defies gravity and travels up the paper towel. How can this happen?
Well, let me introduce you to an interesting concept that causes water to do some really cool stuff. That concept is surface tension. Surface tension is simply a force that exists between two substances, usually a liquid and a surface, such as water and glass. That force can either cause the liquid to be repelled from the surface or attracted to the surface. The above example of water defying gravity and travelling up the paper towel is an example of surface tension in which water is attracted to the paper towel surface.
A good example of a repelling surface is a newly waxed car. The wax on the car is designed to repel water. So what happens when water gets on the car? It beads up into nearly perfect spheres and rolls off like a really fast water wheel. This happens because the water is repelled by the wax. When a water drop hits the car surface, it wants to touch the waxy surface as little as possible and it does this by forming a sphere (draw a picture of a sphere on a surface labeled "wax"). 
Now let's think about another type of surface, one that attracts water, such as wood. If you dip a piece of raw, untreated wood into water and pull it out, you will see that the entire surface has blobs of water all over it. These blobs are a different shape that water is able to make on a surface. The blob shape allows the water drop to touch the surface as much as possible because it is attracted to it (draw a picture of a blob of water on a surface labeled "wood"). Different surfaces have different attractions and repulsions to water. The level of these attractions and repulsions is called surface tension. If we add a treatment to the wood we can change its behavior with water. For example a newly painted wooden deck sheds (repels) water instead of becoming soaked. Chemical engineers design paints and stains to interact with water that way. What shape would you expect water droplets to be on a newly painted deck? (More round than flat.)
In today's activity, Tension Racers, (write this on the board) we will see how water acts on different types of surfaces. A surface that repels water, or a surface with high surface tension, makes a water drop form a sphere-like shape that acts as a really fast wheel that we can use to race water drops down a ramp (draw a sketch of an inclined ramp with a sphere traveling down it). On surfaces with less surface tension, the water forms shapes less and less like spheres and more like blobs. A blob is like a flat wheel — the water moves much slower down a ramp, or not at all (draw a sketch of a blob barely moving down an inclined ramp). Let's get started.
Procedure
Background
This activity conveys how different levels of surface tension affect a water drop's ability to roll down a surface. If a high level of surface tension exists between the water and the surface, the water drop becomes more rounded, making it a better wheel. This principle is illustrated in Figure 2. When a surface is highly non-polar the water molecule is nearly perfectly spherical, when the surface is moderately non-polar the water molecule is more hemi-spherical, and when the surface is weakly non-polar the water molecule tends to spread across the surface.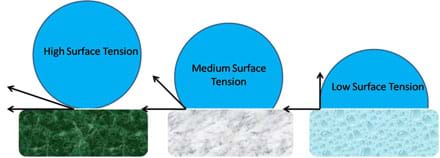
Before the Activity
- Gather materials.
- Divide the class into teams of four students each.
With the Students
- To make the basic "racetrack," cut materials to equal dimensions; 12–24 inches is a good length range. For non-rigid racetrack materials (wax paper, plastic wrap, foil, etc.), wrap them around scrap pieces of stiff plastic or cardboard. Let your imagination guide you and attempt to explore a diversity of materials.
- To prepare for making distance measurements, use a pen or marker to make segmented markings on the materials every centimeter.
- Prop the racetracks on another object, such as a box or wall, to achieve an incline; see Figure 1. Be sure that each track is at the same angle. Emphasize to students that the angle of the track is a variable of the experiment that we want to control. Secure tracks with modeling clay.
- Take a moment for material discussion and prediction, as described in the Assessment section.
- Race water drops down the tracks of different materials. Have students collect data and try a few ways to take measurements (they will need length in cm and time in seconds):
- Simultaneously drop four water drops at the top of each track and see which is fastest.
- Measure how long it takes each water droplet to complete its racetrack.
- Measure how long it takes each water droplet to travel a fixed distance (a subset of its racetrack).
- Measure the distance a water droplet travels in a fixed amount of time (such as two seconds).
- Run numerous races, varying droplet size, height of water droplet release, etc., to see the impact on race results. Again, emphasize to students that these parameters represent different variables of the experiment. To best understand the effects of surface tension, the variables should be constant for each set of trials in order to test the different materials of the racetracks.
- Have students calculate the speeds of the water drops on each track in cm/second. Compare speeds for different materials.
- Have students graph their data and compare to their predictions, as described in the Assessment section. What conclusions can be made? How does this relate to surface tension? Which surface exhibits the highest (or lowest) level of surface tension with water?
- Working in their groups, have students act as engineering teams designing roofs, as described in the Assessment section. Have groups report back to the class their recommendations and answers to the questions.
Vocabulary/Definitions
surface tension: A property of the surface of a liquid that causes an attractive or repulsive force between the liquid and another surface.
Assessment
Pre-Activity Assessment
Predict the Race: Address the different types of materials that are involved in the activity and let students discuss which surfaces they think will enable the water to "race" faster. Have students predict the outcome of the race and provide reasoning for their answers. As time permits, discuss what other conditions or factors may influence how fast the water droplets travel. For example, dust on the surface, water droplet size, and height at which the water is dropped are all variables that can affect race outcome.
Activity Embedded Assessment
Data Collecting: Have the students collect data from the experiment, obtaining length in cm and time in seconds. They could collect the time it takes for the water drop to reach a certain distance or how far the drop moved in a certain amount of time. They could also repeat each race and change droplet size and distance the water is dropped from, which represent control variables of the experiment, to see how that affects the race outcome.
Post-Activity Assessment
Put It All Together: Have students graph their data for each track type and see how well their predictions hold. The students should come to the conclusion that some surfaces (such as raw wood and paper) do not allow the water to move fast or far while other surfaces(such as plastic or wax paper) allow the drop to move fast and far. Discuss how this relates to surface tension and have them come to some conclusions about the relative surface tension between each surface.
Let's Design: Divide the class into engineering "research" teams that have each been "hired" to design the roof of a house. After some discussion and work time, have groups report back to the class their recommendations, which include answers to the following questions.
- What aspects of the roof will influence how well water flows off it?
- Of the materials just tested, which do you recommend as best to prevent water from building up on a roof during a storm?
- Why did you choose that material?
- Is it best to have the roof at an angle? Why?
- Why would you want a roof surface from which water "rolls" off easily?
- For what other applications might you use a material like this?
- Bonus: For what applications might we want a material that does NOT allow water to "roll" off easily?
Troubleshooting Tips
If water droplets are not racing properly, wipe down each of the surfaces. Dust and other particles can severely impede droplet racing.
As necessary, add food coloring to the water to make the drops easier to see.
To speed up the racing droplet, drop the water from a higher distance to give it more force as it hits the surface.
Activity Extensions
Have students suggest and test different types of materials as water droplet racetracks. Based on their previous experiments with the basic materials, have them predict the outcome with the new materials.
Have students further investigate how different types of fluids have different degrees of surface tension on a similar surface. Let students choose to race alternative fluids, such as oil or alcohol.
Activity Scaling
- For lower grades (grades 3-4), it may be more useful to conduct the activity as a visual demonstration in which students observe and discuss how different surface materials change the way water acts.
- For upper grades (grades 5-7), have students conduct the activity extensions.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students are presented with the concepts of wetting and contact angle. They are also introduced to the distinction between hydrophobic and hydrophilic surfaces. Students observe how different surfaces are used to maintain visibility under different conditions.

Students are presented with a short lesson on the difference between cohesive forces (the forces that hold water molecules together and create surface tension) and adhesive forces (the forces that causes water to "stick" to solid surfaces. Students are also introduced to examples of capillary action...

Students are presented with the question: "Why does a liquid jet break up into droplets?" and introduced to its importance in inkjet printers. A discussion of cohesive forces and surface tension is included, as well as surface acting agents (surfactants) and their ability to weaken the surface tensi...
Copyright
© 2014 by Regents of the University of Colorado; original © 2011 College of Engineering, University of South FloridaContributors
Samuel DuPont; Ryan CatesSupporting Program
STARS GK-12 Program, College of Engineering, University of South FloridaAcknowledgements
This curriculum was developed by the USF Students, Teachers and Resources in Sciences (STARS) Program under National Science Foundation grant numbers DGE 0139348 and DGE 0638709. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: April 22, 2022






User Comments & Tips