Quick Look
Grade Level: 10 (9-12)
Time Required: 30 minutes
(5 minutes for preparation; 5-10 minutes for performing activity and explanation; 5 minutes for clean-up)
Expendable Cost/Group: US $1.00
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Physical Science, Science and Technology

Summary
This activity is an easy way to demonstrate the fundamental properties of polar and non-polar molecules (such as water and oil), how they interact, and the affect surfactants (such as soap) have on their interactions. Students see the behavior of oil and water when placed together, and the importance soap (a surfactant) plays in the mixing of oil and water—which is why soap is used every day to clean greasy objects, such as hands and dishes. This activity can easily be scaled to meet any desired level of difficulty.Engineering Connection
The properties of and interactions between polar and non-polar molecules have long been studied and applied in engineering science. Chemical engineers must understand the properties of oil and water and the role that surfactants play in how they act together. The ability to mix such molecularly different substances is extremely important. For instance, engineers often use surfactants to remove oily waste from polluted environments, such as waste dumps and oil spills.
Learning Objectives
After this activity, students should be able to:
- Explain that oil and water differ in polarity and because of this difference they do not mix favorably, allowing water to form a phase-separated sphere in oil.
- Explain that when soap, a type of surfactant, is added to the oil-water system, the surface tension between them is reduced and mixing occurs.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
DCI.PS1.A.9-12.1.
Each atom has a charged substructure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
DCI.PS1.A.9-12.3.
The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
SEP.12.9-12.4.
Use mathematical representations of phenomena or design solutions to describe and/or support claims and/or explanations.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Florida - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
Florida - Science
-
Demonstrate and explain that mixtures of solids can be separated based on observable properties of their parts such as particle size, shape, color, and magnetic attraction.
(Grade
5)
More Details
Do you agree with this alignment?
-
Recognize that atoms are tiny particles in materials, too small to see.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- test tube or other thin-walled, tall and clear vessel
- enough oil to fill the vessel nearly to the top (such as any cooking oil)
- eye dropper
- several 4-8 oz (118-236 mL) cups
- water (enough to fill ¾ of each cup)
- food coloring (a couple of drops per water cup; water-based food coloring recommended)
- liquid dish soap (a few mL at most)
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/usf_surfactants_act2] to print or download.Pre-Req Knowledge
Knowledge of polar, non-polar molecules and the chemistry responsible for repulsive and attractive (non-mixing and mixing) forces in fluid systems, and an understanding of the concept of surface tension.
Introduction/Motivation
Show students Figure 1 either by overhead projection or handouts. The same image is provided in the attached Surfactant Man Visual Aid. Lead a class discussion that includes a short explanation of the polar properties of oil and water that prevent them from mixing, and soap's ability to lower surface tension. As needed, provide a brief review of surface tension. See associated lesson for extensive background information.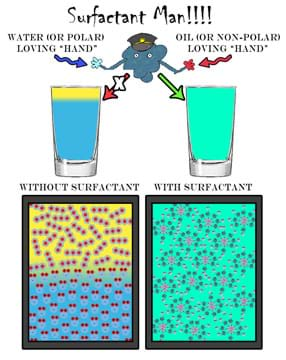
Procedure
Background
This activity helps students visualize the interactions of polar and non-polar molecules and the concept of surface tension.
Oil is hydrophobic, "water fearing," and when water is added to an oil bath it forms into spheres that fall to the bottom due to the forces of gravity. This result conveys two messages: oil and water do not mix and when in contact, they configure their interface to limit contact area (the surface area between the two substances) and since water is denser than oil, water relocates to the bottom of the container.
With the addition of a surfactant to an oil-water system, it is immediately evident that this liquid has unique properties, as it does not fall through the oil in spheres but does so in a more disordered, stream-like fashion. After adequate time for diffusion (natural mixing of the liquids), the effects of lowered surface tension are visually reinforced as the oil and water interfaces become less defined. Show students the general cartoon explanation provided in Figure 1 that uses "surfactant man" to aid in understanding what is occurring at the molecular level.
As suits the level of the students, have students do the calculations or do as a class, to compare the surface areas of a sphere and cube, and circle and square. Refer to the associated lesson for extensive background information on surfactants.
Before the Activity
- Gather materials.
- Divide the class into teams of four students each.
- Conduct the pre-activity assessment discussion, as described in the Assessment section. Have students make their experiment hypotheses and predictions.
With the Students
- Fill the vessels with oil.
- Mix a few (2 or 3) drops of food coloring with 10 mL of tap water. Repeat this step for as many different colors as you want to use.
- Place a drop of dyed water from each of the colored aliquots into the oil vessel.
- Note the behavior of the water as it falls to the bottom of the oil system.
- The shape it forms while falling through the oil.
- The fact that it falls to the bottom.
- The speed at which it falls.
- What happens when the water reaches the bottom?
- Add a few more drops and continue to observe.
- What happens to all of the water drops when they reach the bottom?
- After several water drops have been added, add a small amount of dish soap to each aliquot of colored water.
- Place a drop of the soapy colored water into the oil vessel.
- Note the behavior of the soapy colored water as it passes through the oil system.
- Is the shape the same as previously seen?
- Does it move through the oil at a different speed?
- What happens when it reaches the bottom?
- How does it affect the previously deposited water drops?
- Observe how the water phase acts as the soapy colored water drops contact the previously deposited colored water drops.
- Conduct the embedded activity assessment about the water bubbles, as described in the Assessment section.
- Discuss with students the importance of a surfactant (such as soap) in real-world applications such as:
- Washing hands
- Removing (or dispersing) oil in large bodies of water (such as ocean oil spills)
- Removing (or dispersing) oil on surfaces to keep traction for vehicles, such as cars on roadways or airplanes on runways
- Lead a class discussion to attempt to explain why water forms spheres in the oil, as opposed to other geometric shapes. (The explanation is below) This might also be accomplished by talking about the shape that bubbles take when blown. Why are they only spherical? Why not other shapes? This is due to surface energy. We won't go into much detail on this subject, but suffice it to say that if a surface is not energetically favorable, then the water is repulsed (it tends toward a spherical shape). Conversely, if the surface energy is favorable, the liquid mixes (it increases its contact with surrounding molecules). Depending on students' math abilities, have them do the following calculations (or do as a class) to compare the surface areas of a sphere and cube, and circle and square.
Surface Area Calculation (Sphere vs. Cube)
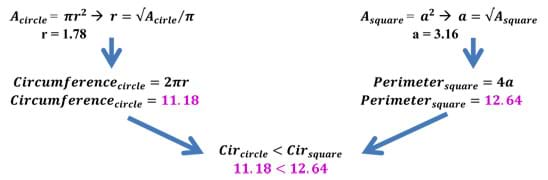
Take a sphere and a cube of the same volume of 10 units. After doing some quick back calculation using equations for volume, we find the following radius and side length (Remember that π = 3.14, approximately). Once the radius and side length have been calculated, use these lengths to determine the amount of surface area a specific shape (in this case a sphere and a cube) has for the same volume (see Figure 2). As we can see, the surface area of the sphere is less than that of a cube of the same volume. In fact, the cube has almost 30% more surface area than the sphere! This example also works with 2D figures. The circle has the least surface area of all shapes. As the calculations show, the "surface area" of a sphere is less than a cube, and this is why water forms spheres!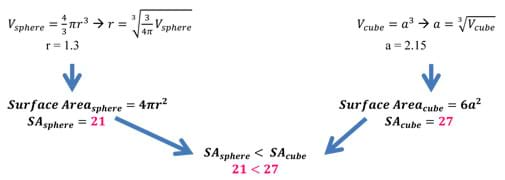
- Conduct the post-activity assessments — further discussion and an engineering problem — as described in the Assessment section.
Vocabulary/Definitions
hydrophilic: A characteristic of having a strong affinity for polar molecules.
hydrophobic: A characteristic of having a strong affinity for non-polar molecules.
interface: A boundary between two systems or phases of matter.
polar: Having globally unequal charge distribution, resulting in charged poles.
sphere: A three-dimensional circle.
Assessment
Pre-Activity Assessment
Why Don't Oil and Water Like One Another?: Before beginning the activity, discuss at length with the students the interaction of oil and water, and have them make their hypotheses for the experiment. Establish the difference of density between the two fluids and the role of gravity. Have students predict how soap will behave when it is introduced into the oil system.
Activity Embedded Assessment
Why Do the Water Bubbles Stack Up When Soap is Not Added?: This would be a great place to discuss and predict the long term activity of the stacked, separate water bubbles. Will they mix over time? Why don't they mix right away? What could be done to force them to mix? What will happen if soap is added to the mixture? (Answer: They will mix over time and their mixing is dependent on the temperature of the system, the viscosity of the fluids, and the degree of difference in the hydrophobicity. Essentially, they do not immediately mix because the water spheres are completely surrounded by oil molecules. When two water spheres get close to one another, a thin layer of oil molecules still exists between them. It takes energy to break this barrier. Energy can be added by mixing or via thermal energy. If left to sit, the thermal energy in the room will eventually allow for breaking of this barrier, but it takes some time.)
Post-Activity Assessment
Is Soap the New Icebreaker?: Discuss with the students why surface tension kept the water bubble separated, why they mixed over time, and the effects that soap played on their interface and surface tension.
Let's Play Engineer: Have students develop a solution to the following engineering problem based on what they have learned about oil and water:
- On airplane runways, a lot of oil accumulates due to normal use of the runway. Excess oil on the runway can be dangerous for planes that are taking off or landing. This is because the oily runway can cause a plane to slip and prevent good acceleration for takeoff, or make it difficult for the plane to slow down during landing. Hot water alone does a poor job of removing the built up oil. What could be a good solution to this problem?
Troubleshooting Tips
When re-using clear vessels, be sure to wash them thoroughly. Any residual surfactant will greatly affect the oil-water interface.
We recommend using water-based food coloring. Some food colorings are alcohol-based, which may slightly alter results. It is strongly recommended that this experiment be performed in advance, for good measure.
Activity Scaling
- This activity is suitable for all grade levels. Adjust the depth of content material from that provided in the associated lesson.
- For upper grades (grades 11-12), this activity can be better applied in the form of a higher level lab in which students perform calculations to find terminal velocity and time for different density spheres and/or different polar solvents in the oil system.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students see how surface tension can enable light objects (paper clips, peppercorns) to float on an island of oil in water, and subsequently sink when the surface tension of the oil/water interface is reduced by the addition of a surfactant; such as ordinary dish soap.

Student teams are challenged to evaluate the design of several liquid soaps to answer the question, “Which soap is the best?” Through two simple teacher class demonstrations and the activity investigation, students learn about surface tension and how it is measured, the properties of surfactants (so...

Students culture cells in order to find out which type of surfactant (in this case, soap) is best at removing bacteria. Groups culture cells from unwashed hands and add regular bar soap, regular liquid soap, anti-bacterial soap, dishwasher soap, and hand sanitizer to the cultures.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 College of Engineering, University of South FloridaContributors
Samuel Dupont; Ryan CatesSupporting Program
STARS GK-12 Program, College of Engineering, University of South FloridaAcknowledgements
This curriculum was developed by the USF Students, Teachers and Resources in Sciences (STARS) Program under National Science Foundation grant numbers DGE 0139348 and DGE 0638709. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: April 11, 2022





User Comments & Tips