Quick Look
Grade Level: 11 (10-12)
Time Required: 2 hours
(two 60-minute class periods)
Lesson Dependency:
Subject Areas: Chemistry, Physics, Science and Technology

Summary
Students experience firsthand one of the most common water treatment types in the industry today, flocculants. They learn how the amount of suspended solids in water is measured using the basic properties of matter and light. In addition, they learn about the types of solids that can be found in water and the reasons that some are easier to remove than others. Encompassing the concepts of force and motion, attraction and repulsion of charged particles, and properties of matter, during the associated activity students see scientific concepts they already understand through the eyes of engineers who apply them to the removal of solids from water via chemical flocculants.Engineering Connection
Almost a billion people do not have access to clean drinking water. Engineers help solve this problem by using flocculants to clean water before and after it is used in households around the world. Several different flocculants are currently used to clean water, and engineers continue to design more effective and less expensive ways to remove particles from water.
Learning Objectives
After this lesson, students should be able to:
- Define reflection, absorption and transmission of light, in terms of matter.
- Explain how the properties of light and matter assist in measuring the water turbidity.
- Identify the two main types of suspended solids found in water.
- Define colloids and explain why they are difficult to remove from water based on size and surface charge.
- Describe flocculants and how they work to remove colloids.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Analyze how an invention or innovation was influenced by its historical context.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Florida - Science
-
Science and Society
(Grade
8)
More Details
Do you agree with this alignment?
-
Understand that scientific investigations involve the collection of relevant empirical evidence, the use of logical reasoning, and the application of imagination in devising hypotheses, predictions, explanations and models to make sense of the collected evidence.
(Grade
8)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/usf_flocculant_lesson01] to print or download.Pre-Req Knowledge
Students should have a general understanding of basic chemistry, specifically the concepts of attraction and repulsion of electrically charged objects.
Introduction/Motivation
We all consume water every day in one form or another. What would happen to us if we did not? Water is vital to our life, in other words, we could not live without it. But where does the water we drink come from? Do we take it right out of the ground and bring it into our houses? What about when we are finished with it, do we just put it right back into the ground? If this were the case, the water we use every day might be too dirty for us to drink and we could get really sick and maybe even die!
When we realized that the quality of water (how clean it is) is just as important as drinking it, we developed tools that help us remove the harmful things that can be in the water when it is taken from the ground. We also developed ways to measure how dirty the water is, so that we can make sure that it meets our needs before it is distributed to us. For this purpose, we use light!
You may recall that objects can either reflect or absorb light. Using this property of matter, we can shine a light through the water and see how much gets through. We call this measurement turbidity. This may be a word you have not heard before, so let us take a look at it. Have you heard the word turbid? It means unclean or full of stuff. So, turbidity tells us how unclean or how much stuff is in the water. Now that we know how dirty the water is, how do we clean it?
One of the first steps in the water treatment process is to remove solids such as sand or dirt that are in the water. We achieve this by using a flocculant. Have you ever heard of the word flocculant? (Write "flocculant" on the board.) Look at the word for a minute. Do you know what a flock is? It is a collection of things, right? From this you may have figured out that a flocculant is something that causes particles and dirt suspended in water to come together. Okay, so now we have the particles together, but what does that do for us? Do you remember how the molecules in liquid behave? They slide around each other, right? While they are doing this, they hit each other sometimes and also hit whatever else is in the water. These collisions are part of the reason that the solids are staying in the water, too. Also, you may remember that heavier objects (say, a lot of particles stuck together) take more force to move than smaller objects (say, one lone particle). These two basic concepts are the reasons that flocculants work. When the particles come together, they get heavier. When they get heavier, the water molecules cannot push them around as easily as they used to, and gravity pulls them to the bottom of the water container. The result is water with less dirt in it!
Lesson Background and Concepts for Teachers
Despite our planet's large amount of water, very little freshwater is available for consumption. To meet the needs of the world's population, people have developed methods to clean water obtained from the ground. Many of these methods are also used to clean water after it is used so that it can be returned to the ground without unwanted impacts, such as pollution. Several contaminant types are found in water including chemicals (such as salts and sugars), microorganisms (such as bacteria and algae), and solids (such as clay and sand). Most of these contaminants are removed in the water treatment cycle. In this lesson, we focus on the removal of solids.
Turbidity—To measure water cleanliness prior to consumption, we use the concept of turbidity. Derived from the word turbid, turbidity is a measurement of the "cloudiness" of water. It essentially tells us the amount of solids in the water.
Turbidity is measured by using light's response to matter. When a light is shined on matter, matter can either reflect or absorb the light. Reflected light bounces off of the matter in many directions, whereas absorbed light gets trapped within. The light that passes through matter is called transmission. See Figure 1 for a diagram of these concepts.
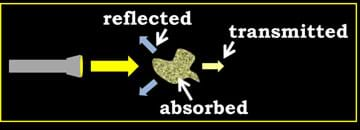
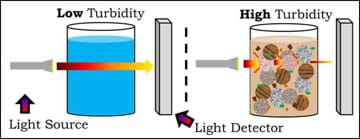
The amount of solids in water can be measured using the property of transmission. The diagram in Figure 2 shows how a system can accomplish this using a light source and a light detector. In the diagram, the water sample on the left has a "low" turbidity, which means not much solid matter is in the water, resulting in a high transmission. When transmission is high, most of the light that is directed through the sample reaches the detector on the other side (note the arrow width of the light detected with the left sample). If lots of solids exist in the water, it has a "high" turbidity, as shown in the right water sample. A high turbidity is found when very little of the light directed through the sample makes it to the detector (note the reduced arrow width reaching the detector on the right side). This occurs when a lot of reflection or absorption occurs due to the presence of matter (solids) in the water.
Types of Suspended Solids—Settling and non-settling are two major types of solids found in water. The combination of these two types makes up the total suspended solids found in water. Settling solids are usually larger particles that are visible with the naked eye and are generally bigger than 1 micrometer (1 μm = 10-6 meters = 0.000001 meters). Examples of settling solids are sand and small rocks. Non-settling solids are smaller particles that are difficult to see without magnification. These range from 1 – 1,000 nanometers (1 nm = 10-9 meters = 0.000000001 meters). Examples of non-settling solids are fine silts and fine clays. Non-settling solids are often responsible for the dirty or cloudy coloring of water, and are also known as colloids.
Colloids are formed when a material, such as fine silt or fine clay, is microscopically suspended throughout another material, in our case, water. Colloidal suspensions have a dispersed phase (the suspended material) and a continuous phase (water).
Colloids are difficult to remove from water for several reasons. First, they are extremely small, making it difficult for them to settle to the bottom of the water on their own. On the other hand, liquid molecules are always in motion. If colloids are in the liquid, the molecules in motion can hit these suspended solids, moving them back and forth. The very small colloids do not require much force to move around and stay in solution. The random movement of these solids (colloids) in water is called Brownian motion. It is the Brownian motion of the solids that keep them suspended in water for so long.
Another reason that colloids take a long time to settle out of water is surface charges. Surface charges can form on a particle in several ways. Before explaining how these surface charges form, let's review the structure of atoms and the concept of bonds.
Flocculants—The tiny size and surface charge of colloids (small charged particles) make them difficult to remove from water, so engineers turn to flocculation. Flocculation is now an essential step in many water purifications systems as a means to remove suspended solids. This essential step is done before filtration in order to avoid clogging. A flocculant is a chemical that can be added to the water to help colloids and any other suspended solids bind together and form heavier particles. The heavier particles then settle to the bottom of the container/tank and the water on the top is drained off.
One of the most common flocculants used in water purification today is aluminum sulfate (also known as alum); its chemical composition is shown in Figure 3.
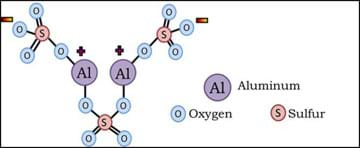
Aluminum sulfate is a white, solid (powder) with a chemical formula of Al2(SO4)3. It is an ideal choice to use as a flocculant because when it dissociates in water, it forms a high amount of charged compounds, as shown in Figure 4.
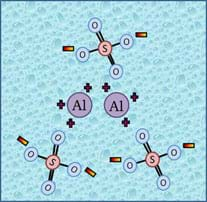
When aluminum sulfate is added to water, it dissociates and forms ions (charged atoms). Colloidal particles with surface charges bind together with these ions and become heavy enough to settle to the bottom of the water. For example, if aluminum sulfate is added to water containing colloidal particles with negative charges, the colloidal particles' negative charges are attracted to the aluminum's positive charges (opposites attract!). This causes the particles to aggregate, increasing in mass, such that the motion in the water is no longer enough to retain the particles in suspension.
Another common flocculant is polyacrylamide. Polyacrylamide is a polymer, meaning it is made of many (poly) parts (mer). The name polyacrylamide, indicates the type of parts that compose the chemical: poly (many) acrylamide (type of parts). The chemical acrylamide has the structure shown in Figure 5.
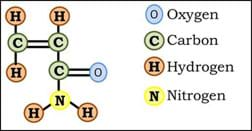
When acrylamide polymerizes (turns into a polymer), a chain of molecules is formed, as shown in Figure 6.
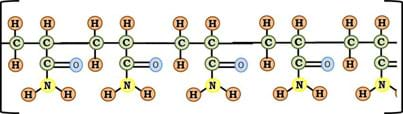
Polymers are useful as flocculants because they are robust molecules and sometimes carry charges. Because they are so large, small particles can get trapped in the curves of the polymer causing them to accumulate a mass heavy enough to prevent their retention in solution.
Many other flocculants are currently used in water treatment today and even more are being studied by engineers and researchers around the world. To review, all flocculants work by increasing mass and causing solids to settle out of water, making it cleaner. As previously discussed, turbidity is a measurement of the amount of solids suspended in water. By letting the water sit for a short period of time prior to treatment, larger settling solids eventually go to the bottom of the container, slightly reducing the turbidity. By using a flocculant, the turbidity, as well as the "dirty" coloring of the water, can be greatly reduced because the smaller solids are also removed.
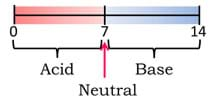
Things That Matter to Flocculants—Several factors affect how flocculants work. By increasing the amount of kinetic energy in a solution through stirring or heating, flocculants have a faster impact on the particles. This phenomenon occurs because as kinetic energy is added to solution, particles move faster and therefore clump with the flocculant at a faster rate. The volume of solution and flocculant can also affect flocculation. Chances are, if a large amount of water is being cleaned, a larger amount of flocculant is needed. The most effective dose may also change depending on the flocculant type, which is often dictated by the available charges or size of the flocculant. Additionally, the water's pH can affect the performance of a flocculant. The pH scale (see Figure 7) is used to determine how acidic or basic the water is. Most flocculants work best at neutral conditions (pH 7) while some work independently of the pH. Have students expand their understanding with the hands-on Things That Matter to Flocculants activity.
Associated Activities
- Things That Matter to Flocculants - Students learn about two commonly used chemical additives (flocculants) that remove suspended solids in water. They collect and clean water from a local pond or river, experimenting with variations in flocculant, stirring and pH.
Vocabulary/Definitions
bond: The chemical interaction between atoms that results in a compound consisting of two or more atoms.
Brownian motion: The random movement of particles in water caused by the contact of water molecules.
colloid: Any material microscopically suspended in another.
dissociation: Causes a solid to dissolve in a liquid (such as salt in water)
electron orbit: The path that an electron follows as it circles around the nucleus (center) of an atom. (Similar to the Earth's orbit around the sun.)
flocs: The clustered particles formed in the presence of a flocculant.
ion: An atom that contains an unequal number of protons and electrons, resulting in a negative or positive charge.
polymer: A large molecule chain that is composed of many small parts.
reflection, absorption and transmission: The phenomena that occur when light hits matter. Reflected light is scattered back from the object; absorbed light energy stays within the object; transmitted light is the light that makes it through.
total suspended solids: The total amount of settling (large) and non-settling (small) solids that are suspended in water.
turbidity: A measure of the "cloudiness" of water (total amount of suspended solids in water). It can be measured by using a light source and a detector.
Assessment
Pre-Lesson Assessment
Discussion: Ask question to get students to think about the upcoming lesson.
- What is the source of the water that comes from the faucets in your homes and school? Was it always that clean? What happens to it after it is dirty and goes down the drain? (Answer: [In most cases...] The water that is distributed to us in our homes and classrooms comes from water treatment plants. There, the water that is collected from groundwater, lakes or other sources is cleaned before being piped to the people living in a community.)
- What sort of methods are used to clean the water before it comes to our faucets? (Students may already know about filters, but they have probably never heard the word "flocculant" before. Bring it up and see if they can figure out what it does based on the root of the word [floc]. Explain that they will learn more about flocculants during the lesson.)
Post-Lesson Assessment
Journal Reflection: Have students discuss and record in their journals the reasons why using flocculants may be beneficial. Suggest they think in terms of filters (they prevent clogging, allowing filters to last longer and therefore lowering filter replacement expenses) and in terms of water (they reduce suspended solids). Also, have students brainstorm about why the use of natural flocculants (which are currently being studied by many researchers) might benefit the world. Discuss the idea that some countries do not have access to water treatments that are as robust those in the US. How might natural flocculants benefit these countries? (Answer: Natural flocculants are found in some plants, such as cactus raket and the moringa tree pod, which are readily available in certain regions of the world. In these cases, community members can simply collect the naturally available flocculant and use it in water treatment processes!)
Quiz: After the lesson, administer the attached Post-Lesson Quiz to gauge student comprehension of the material covered.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students learn the fundamentals of using microbes to treat wastewater. They discover how wastewater is generated and its primary constituents. Microbial metabolism, enzymes and bioreactors are explored to fully understand the primary processes occurring within organisms.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 College of Engineering, University of South FloridaContributors
Audrey Buttice; Marissa H. ForbesSupporting Program
STARS GK-12 Program, College of Engineering, University of South FloridaAcknowledgements
This curriculum was developed by the USF Students, Teachers and Resources in Sciences (STARS) Program under National Science Foundation grant numbers DGE 0139348 and DGE 0638709. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: June 7, 2019




User Comments & Tips