Quick Look
Grade Level: 10 (9-12)
Time Required: 45 minutes
Expendable Cost/Group: US $2.00 The activity also requires some non-expendable (reusable) items; see the Materials List.
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Science and Technology
NGSS Performance Expectations:

| HS-ESS3-4 |
| HS-PS1-4 |
Summary
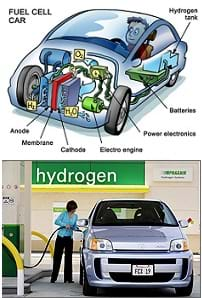
Through this lab, students are introduced to energy sciences as they explore redox reactions and how hydrogen fuel cells turn the energy released when hydrogen and oxygen are combined into electrical energy that can be read on a standard multimeter. They learn about the energy stored in bonds and how, by controlling the reaction, this energy can be turned into more or less useful forms.Engineering Connection
In the age of ever multiplying personal devices, it is important for all young people to understand the basic principles behind battery development and charging systems. A battery is only as clean as the source that charges it. When engineers design batteries they consider many design constraints. Apart from energy density, engineers also consider size, safety, mobility, volatility, environmental impact and efficiency. Creating a useful battery takes the balancing of many different costs and benefits.
Learning Objectives
After this activity, students should be able to:
- Evaluate the environmental impact of energy sources using conservation of energy concepts.
- Demonstrate a basic understanding of the science behind storing and releasing chemical potential energy.
- Explain how engineers apply these scientific concepts to solve real-world problems.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ESS3-4. Evaluate or refine a technological solution that reduces impacts of human activities on natural systems. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design or refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Scientists and engineers can make major contributions by developing technologies that produce less pollution and waste and that preclude ecosystem degradation. Alignment agreement: When evaluating solutions it is important to take into account a range of constraints including cost, safety, reliability and aesthetics and to consider social, cultural and environmental impacts.Alignment agreement: | Feedback (negative or positive) can stabilize or destabilize a system. Alignment agreement: Engineers continuously modify these technological systems by applying scientific knowledge and engineering design practices to increase benefits while decreasing costs and risks.Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model based on evidence to illustrate the relationships between systems or between components of a system. Alignment agreement: | A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart. Alignment agreement: | Changes of energy and matter in a system can be described in terms of energy and matter flows into, out of, and within that system. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Energy cannot be created nor destroyed; however, it can be converted from one form to another.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Energy can be grouped into major forms: thermal, radiant, electrical, mechanical, chemical, nuclear, and others.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Evaluate ways that technology can impact individuals, society, and the environment.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Develop a solution to a technological problem that has the least negative environmental and social impact.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Michigan - Science
-
Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Evaluate or refine a technological solution that reduces impacts of human activities on natural systems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- Brownlee electrolysis apparatus, such as these available at Amazon; prices vary, $30+
- baking soda (or vinegar), as an electrolyte (perhaps two or three teaspoons of baking soda)
- 2 platinum electrodes with fitted rubber stoppers, such as two for $56 from Fisher
- solar panel (or battery eliminator), such as this medium 6V 2W solar panel for $35 from Adafruit
- multimeter or voltage meter
- Redox Reactions and Alternate Energy Solutions Handout, one per student
- safety glasses or goggles, one pair per student
To share with the entire class:
- electrolysis setup for class demonstration
- matches
- safety glasses or goggles
- distilled water
- (optional) AA battery
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/mis_redox_activity1] to print or download.Introduction/Motivation
(Begin by demonstrating the combustion of hydrogen to pique students' curiosity. As students walk in, have an electrolysis apparatus producing gas into a single test tube. The hydrolysis apparatus can be the same as the Brownlee apparatus students use in the activity, but for a more violent reaction, collect the gas in a single tube rather than two separate tubes.)
Take a look at the apparatus in the front of the room. I have an electrical power source flowing current through this solution. What's happening? (Expect students to recognize that bubbles are being created.)
Can anyone guess—what are these bubbles made of? Think about what water is made of. (Expect students to recognize that the bubbles are hydrogen and oxygen.)
Chemical bonds are able to store and release energy. When water is turned into oxygen and hydrogen, is energy being stored or released? (Expect students to recognize that it takes energy to separate oxygen and water, so separating the two stores energy.)
If separating hydrogen and water takes energy, what happens if they are put back together? (Expect students to recognize that this releases energy.)
Who has heard of hydrogen fuel cells? (See what students know.) Hydrogen fuel cell technology relies on the energy that is released when hydrogen and oxygen are combined to create water. This energy can be transformed into useful energy, as you will see during your lab, and non-useful energy, which I will demonstrate in just a minute. Unfortunately for us, conservation of energy prevents us from making free energy. In order to release energy as hydrogen and oxygen combine to make water, we must first add energy to separate water into hydrogen and oxygen.
The apparatus at the front of the room is performing what's called electrolysis. Electrolysis uses electrical energy passed through an electrolyte solution to split water molecules into hydrogen and oxygen. One way of releasing energy by combining the two gases is through combustion.
(At this point, remove the test tube filled with the H2 and O2 mixture and use a match to light it. This causes a very small explosion.)
As you can see, quite a bit of energy was released when hydrogen and oxygen combined to make water. In this instance, the energy produced was not particularly useful. Combustion produces water and heat energy. In order to be useful, combustion must be controlled in a complicated machine such as an internal combustion engine.
Your design problem for today's lab is how to control what would normally be a very violent reaction. If you were engineers, finding a solution for this problem would help you create a more controlled energy source.
Procedure
Background
When water is separated into oxygen and hydrogen, energy is stored. When oxygen and hydrogen are recombined to make water, energy is released. The reaction of oxygen and hydrogen is very violent and wastes a lot of energy as heat. In this activity, students create a device that performs this redox reaction in a way that the energy can be harnessed in a more useful way. Students then apply this experience as they reflect on the decisions that engineers must make regarding environmental impact and fuel cell design.
To start the activity, demonstrate the separation of water into hydrogen and oxygen by setting up a Brownlee apparatus so that it produces oxygen and hydrogen gas into a single test tube rather than two. After a small amount of gas has been produced, remove the test tube and ignite it to produce a small explosion. Only a small amount of gas is needed and a large amount can be dangerous.
Students use the augmented Brownlee apparatus (explained below) to produce hydrogen and oxygen gas using a solar panel as the energy source. Once the hydrogen and oxygen gas reach the platinum electrodes at the top of the tube, the solar panel is disconnected and the voltage meter is attached across the platinum electrodes. The voltage meter should show a voltage across these electrodes (just like a battery).
For reference, refer to the following videos that show and describe the apparatus setups:
- Fuel Cell Student Demonstration (4:46 minutes): https://youtu.be/bnzpjP7mSQY
- Fuel Cell Teacher Demonstration (3:56 minutes): https://youtu.be/qvwa5ODb-1A
Through this activity, students are asked to evaluate the use of hydrogen fuel cells—a technological solution that can be a way of reducing human impact on our planet. Students are meant to see that it takes energy to split hydrogen and oxygen to make the fuel cell and that energy has to come from somewhere. If it's coming from coal, for instance, it doesn't reduce our impact on the environment.
Before the Activity
- Make copies of the Redox Reactions and Alternate Energy Solutions Handout, one per student.
- Use of the solar panels requires a sunny day and access to the outdoors. Alternatively, to conduct the activity as an indoor experiment, use a battery eliminator instead of a solar panel.
- In the Brownlee electrolysis apparatus, replace the test tubes with straight glass tubes fitted with platinum electrodes. Use a rubber stopper to seal the electrodes in place. If glass tubes are unavailable, use old test tubes cut using a file and polished using a Bunsen burner. Make sure that they have no sharp edges before inserting the rubber stoppers. As another alternative, use clear plastic tubing, which is available online.
- Prepare an out-of-the-way table or counter space where the group apparatuses can be left undisturbed overnight.
- Prepare an electrolysis demonstration to have running at the start of the class, as described in the Introduction/Motivation section. Use a hydrolysis apparatus that is the same as the Brownlee apparatus students use in the activity, but collect the gas in a single tube rather than two tubes, for a more dramatic reaction.
With the Students Overview
- As students enter the classroom, with the demo producing gas into a test tube, proceed to present the Introduction/Motivation content, including the demo "pop" explosion as well as an introduction to the activity engineering design problem: To create a device to control what would normally be a very violent reaction, so that the energy can be harnessed in a more useful way, creating a more controlled energy source.
- Divide the class into small teams of approximately four students per group.
- Pass out a handout to each student.
- Direct the groups to follow the handout instructions for the lab, which are also listed below:
- As groups are engaged in the activity, circulate to make sure all apparatuses are functioning and ask probing discussion questions, such as those suggested in the Assessment section.
Student Lab Instructions: Part 1
- Place one of the tubes in the water so that the water covers the top of the tube.
- Using light pressure secure a rubber stopper with an electrode in it inside the top of tube so that the water is contained inside the tube. Be careful not to break the tube when inserting the rubber stopper.
- Repeat this process with the other tube.
- Secure both tubes to the apparatus so they are held vertically about 2 cm above the bottom of the container.
- Carefully add a small amount of baking soda to the water to serve as an electrolyte.
- Place one bent electrode at the bottom of each tube so the metal tip is pointing upward into the tube.
- Attach the positive lead of the solar panel to one electrode and attach the negative lead of the solar panel to the other electrode. (If done as an indoor experiment, use a battery eliminator instead of a solar panel.)
- The apparatus should resemble Figure 1. When given permission by the teacher, carefully walk the apparatus outside so that the solar panel is in the sunlight.
- Let the system run until the electrode at the top of each tube is covered with gas, then detach the leads.
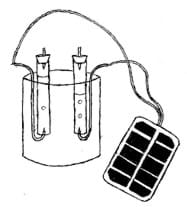
Figure 1. Lab apparatus setup showing the connection to the solar panel.
Student Lab Instructions: Part 2
- Place the voltage meter across the electrodes on the top of the tubes. Platinum works as a catalyst for the reaction; be sure that each electrode is completely covered in gas. The apparatus should resemble Figure 2.
- With the multimeter attached to the electrodes, place the power cell in a location where it will not be disturbed. Mark the gas levels in each tube and check the tube in the morning.
- To conclude, have students answer the handout questions and turn them in for grading. If time permits, discuss the questions and answers as a class.
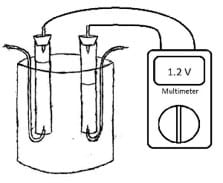
Figure 2. Lab apparatus setup showing the connection to the multimeter.
Vocabulary/Definitions
battery: A device that stores chemical potential energy, which can then be transformed into electrical energy.
chemical potential energy: Energy stored in chemical bonds that can be turned into other forms of energy. When a reaction results in a lower energy level, that energy must be released, usually in the forms of heat, light or mechanical energy. This is how batteries are able to power lights and run motors.
electrolysis: The use of electrical current to break water (H2O) into oxygen and hydrogen gas. This reaction is not spontaneous, meaning it takes energy to happen. An inexpensive way to produce pure hydrogen and oxygen.
oxidation: When electrons are added to an atom or molecule during a chemical reaction. This is the "receiving end" of an oxidation/reduction reaction.
reduction: When electrons are removed from an atom or molecule during a chemical reaction. This is the "giving end" of an oxidation/reduction reaction.
voltage: A measure of how much energy per unit of charge that passes from one electrode to another electrode; for example, a 9 volt battery can provide 9 joules of energy for every coulomb of charge that passes from its positive electrode to its negative electrode. In the case of redox reactions, think of voltage as the amount of energy released per reaction that happens.
Assessment
Pre-Activity Assessment
Questioning: As incorporated into the Introduction/Motivation section, while running the demo apparatus in the front of the room, ask students the following questions to assess their prior knowledge:
- Take a look at the apparatus in the front of the room. I have an electrical power source flowing current through this solution. What's happening? (Expect students to recognize that bubbles are being created.)
- Can anyone guess: What are these bubbles are made of? Think about what water is made of. (Expect students to recognize that the bubbles are hydrogen and oxygen.)
- Chemical bonds are able to store and release energy. When water is turned into oxygen and hydrogen, is energy being stored or released? (Expect students to recognize that it takes energy to separate oxygen and water, so separating the two stores energy.)
- If separating hydrogen and water takes energy, what happens if they are put back together? (Expect students to recognize that this releases energy.)
- Who has heard of hydrogen fuel cells?
Activity Embedded Assessment
Inquiry Questions: Circulate around the classroom making sure all the apparatuses are functioning and asking probing questions, such as the following:
During Part 1:
- What can you do to speed up the reaction? (Answer: Tilt the solar panel so that it faces directly at the sun.)
- Why do you think we are putting the gases into different tubes? (Answer: So we can control the reaction. Later in this activity, we will force the charge to flow through a circuit rather than having the chemical reaction manifest as a violent explosion.)
- Why do you think we use baking soda in the water rather than just pure water? (Answer: Baking soda acts as an electrolyte, enabling us to charge to flow through the solution and catalyze the hydrolysis.)
- Would the reaction still happen if the tubes were not connected by the electrolyte solution? (Answer: No, charge would not be able to flow from one electrode to the other so no reaction would take place.)
During Part 2:
- An AA battery reads 1.5 V on the multimeter. How does the voltage of your fuel cell compare to the voltage of an AA battery? (Answer: It is a little bit smaller, between 1.0V and 1.5V. Optional: Have handy a charged AA battery so students can compare.)
- Current is how quickly charge flows through a circuit. Do you think your fuel cell can produce much current? (Answer: The gas levels do not decrease that quickly so it probably cannot produce much current.)
- (If students are familiar with electronics) How could we combine the cells to produce more voltage or more current? (Answer: For more voltage, connect them in series; for more current, connect them in parallel.)
- (If students get done early) Put two fuel cells together in series and measure the voltage across both. What do you get? (Answer: Expect voltage to double with twice the number of cells.)
- Why do you think we use platinum for the electrodes? (Answer: They catalyze the reverse reaction.)
Post-Activity Assessment
Handout Questions: Have students complete the Redox Reactions and Alternate Energy Solutions Handout by answering the questions at the end. Review their answers to gauge their depth of comprehension. Example answers are provided in the Redox Reactions and Alternate Energy Solutions Handout Answer Key. If time permits, discuss the questions and answers as a class.
Safety Issues
The combustion of hydrogen and oxygen is a violent reaction. To create a large enough explosion to impress students without becoming dangerous, produce 2-4 ml of gas in the test tube, which is enough to make a sharp "pop" sound. If too much hydrogen is created, simply angle the tube to permit some gas to escape. Then, for the demonstration, hold the tube perpendicular to the class. Wear safety glasses and do not aim test tubes containing these gases toward anyone or anything flammable.
Activity Extensions
Instead of answering the handout questions, require students to complete a full lab report in a format of the teacher's specification.
Have students continue using the lab setup through the use of batteries to determine if electrolysis is influenced more by voltage or current by placing batteries in parallel and series and taking data on gas produced during a given time period.
Have students research different types of energy storage systems that have been developed. Direct them to focus on battery chemistry especially the difference between rechargeable and one-time use batteries, as well as the memory effect that plagues older chargeable batteries, but not newer lithium polymer batteries.
Have students use the electrolysis apparatus to collect hydrogen in an upright pop bottle. Direct students use stoichiometric principles to determine what amount of hydrogen would make the pop bottle shoot the highest.
Additional Multimedia Support
Refer to the following videos that show and describe the apparatus setups:
- Fuel Cell Student Demonstration (4:46 minutes): https://youtu.be/bnzpjP7mSQY
- Fuel Cell Teacher Demonstration (3:56 minutes): https://youtu.be/qvwa5ODb-1A
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

This lab exercise exposes students to a potentially new alternative energy source—hydrogen gas. Student teams are given a hydrogen generator and an oxygen generator. They balance the chemical equation for the combustion of hydrogen gas in the presence of oxygen.
Copyright
© 2015 by Regents of the University of Colorado; original © 2012 Michigan State UniversityContributors
Alexander RobinsonSupporting Program
Robotics Engineering for Better Life and Sustainable Future RET, College of Engineering, Michigan State UniversityAcknowledgements
The contents of this digital library curriculum were developed through the Robotics Engineering for Better Life and Sustainable Future research experience for teachers under National Science Foundation RET grant number CNS 1300794. However, these contents do not necessarily represent the policies of the NSF and you should not assume endorsement by the federal government.
Last modified: October 9, 2020



User Comments & Tips