Quick Look
Grade Level: 4 (3-5)
Time Required: 45 minutes
Expendable Cost/Group: US $0.00 This activity requires use of a non-expendable (reusable) LEGO® MINDSTORMS® robot kit; see the Materials List for details.
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry
NGSS Performance Expectations:

| 3-5-ETS1-3 |
| 5-PS1-3 |

Summary
Students explore materials engineering by modifying the material properties of water. Specifically, they use salt to lower the freezing point of water and test it by making ice cream. Using either a simple thermometer or a mechatronic temperature sensor, students learn about the lower temperature limit at which liquid water can exist—such that even if placed in contact with a material much colder than 0 degrees Celsius, liquid water does not get colder than 0 °C. This provides students with an example of how materials can be modified (engineered) to change their equilibrium properties. They observe that when mixed with salt, liquid water's lower temperature limit can be dropped. Using salt-ice mixtures to cool the ice cream mixes to temperatures lower than 0 °C works better than ice alone.Engineering Connection
Using instruments such as thermometers and mechatronic sensors to gather data is an important skill for engineers who conduct research as part of the engineering design process. Materials engineers experiment to modify material properties to yield certain desirable characteristics to meet client and product needs.
Learning Objectives
After this activity, students should be able to:
- Take measurements and organize data in a simple chart.
- Describe the material properties of water.
- Describe how material property modification can be used to make materials useful for specific tasks.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
3-5-ETS1-3. Plan and carry out fair tests in which variables are controlled and failure points are considered to identify aspects of a model or prototype that can be improved. (Grades 3 - 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation collaboratively to produce data to serve as the basis for evidence, using fair tests in which variables are controlled and the number of trials considered. Alignment agreement: | Tests are often designed to identify failure points or difficulties, which suggest the elements of the design that need to be improved. Alignment agreement: Different solutions need to be tested in order to determine which of them best solves the problem, given the criteria and the constraints.Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
5-PS1-3. Make observations and measurements to identify materials based on their properties. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Make observations and measurements to produce data to serve as the basis for evidence for an explanation of a phenomenon. Alignment agreement: | Measurements of a variety of properties can be used to identify materials. (Boundary: At this grade level, mass and weight are not distinguished, and no attempt is made to define the unseen particles or explain the atomic-scale mechanism of evaporation and condensation.) Alignment agreement: | Standard units are used to measure and describe physical quantities such as weight, time, temperature, and volume. Alignment agreement: |
Common Core State Standards - Math
-
Solve multistep word problems posed with whole numbers and having whole-number answers using the four operations, including problems in which remainders must be interpreted. Represent these problems using equations with a letter standing for the unknown quantity. Assess the reasonableness of answers using mental computation and estimation strategies including rounding.
(Grade
4)
More Details
Do you agree with this alignment?
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Asking questions and making observations helps a person to figure out how things work.
(Grades
K -
2)
More Details
Do you agree with this alignment?
State Standards
Delaware - Science
-
Plan and carry out fair tests in which variables are controlled and failure points are considered to identify aspects of a model or prototype that can be improved.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Make observations and measurements to identify materials based on their properties.
(Grade
5)
More Details
Do you agree with this alignment?
New York - Science
-
Plan and carry out fair tests in which variables are controlled and failure points are considered to identify aspects of a model or prototype that can be improved.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Make observations and measurements to identify materials based on their properties.
(Grade
5)
More Details
Do you agree with this alignment?
Virginia - Science
-
The student will investigate and understand the unique properties and characteristics of water and its roles in the natural and human-made environment. Key concepts include
(Grade
6)
More Details
Do you agree with this alignment?
-
the properties of water in all three phases;
(Grade
6)
More Details
Do you agree with this alignment?
Materials List

Each group needs:
- a thermometer OR a LEGO MINDSTORMS EV3 Intelligent Brick with an attached LEGO MINDSTORMS Temperature Sensor (the EV3 Intelligent Brick at https://education.lego.com/en-us/products/lego-mindstorms-education-ev3-intelligent-brick/45500); the MINDSTORMS temperature sensor at https://education.lego.com/en-us/products/lego-mindstorms-education-ev3-temperature-sensor/9749
- 1 gallon-size plastic bag
- ice cubes, enough so the gallon-size plastic bag is about one-quarter filled
- one-quarter cup ice cream mix placed in a 1 quart-sized Ziploc® bag (see below for ingredients to make the ice cream mix )
- wooden stirring spoon
- paper towel
- paper and pencil
- watch or stopwatch, to measure two-minute intervals (or use classroom wall clock)
- plastic spoon, one per student
- 12 Dixie cups, three per student
In addition, prepare the following supplies for the three different group types:
- give the "no salt" groups nothing extra
- give the "some salt" groups each a plastic baggie containing one-quarter cup salt
- give the "lots of salt" groups each a plastic baggie containing 1.5 cup salt (or more)
To make the ice-cream mix (makes 17 single-bag [¼ cup] servings):
- 2 cups half & half
- 2 cups heavy cream
- ¼ cup powdered sugar
- 1 teaspoon vanilla extract
To share with the entire class:
- classroom board or overhead projector
Alternative: LEGO MINDSTORMS NXT Set:
Note: This activity can also be conducted with the older (and no longer sold) LEGO MINDSTORMS NXT set instead of EV3; see below for those supplies:
- a thermometer OR a LEGO MINDSTORMS NXT Intelligent Brick with an attached LEGO NXT Temperature Sensor
- computer loaded with the NXT 2.1 software
Pre-Req Knowledge
Familiarity with the properties of water. An understanding that cold materials can cool warmer materials by contact.
Introduction/Motivation
Pure water always freezes at the same temperature, zero degrees Celsius (0 °C). Water that is above zero degrees Celsius is liquid, but if you cool water to below zero degrees Celsius, even by just a single degree, it becomes solid—what we know as ice.
This change from liquid to solid is known as a "phase transition," meaning the change from one "phase" of matter, such as a solid, liquid or gas, to another phase. The temperature at which the phase transition between liquid and solid occurs is called the "freezing point."
From your experiences in daily life, you are probably already familiar with the freezing phase transition of water. For example, if you place an ice cube tray filled with liquid water into the freezer, what will happen? (Listen to student responses.) That's right, you know that you will get solid ice cubes once the water becomes "cold enough." The water becomes "cold enough" because the freezer is colder than zero degrees Celsius. By putting the liquid water in the freezer, you allow it to "equilibrate," meaning it eventually reaches the same temperature as the freezer.
Making liquid water turn into solid ice cubes is an example of materials engineering, since you have modified a materials properties of water with an application in mind—perhaps using the ice cubes to cool a drink!
With this in mind, can you think of any way I can cool water to below zero degrees Celsius, and have it remain liquid? (Listen to student responses.) Well, there is a way to get water colder than zero degrees Celsius—you can get water colder than zero degrees Celsius by engineering the properties of water! Changing the properties of water is pretty easy—you can change tap water into salt water, and salt water has different properties than tap water. If you have water that is mixed with a LOT of salt, it can still be liquid at as low as -20 degrees Celsius instead of zero degrees Celsius. This is called a modification of the freezing point of water. Cool, right?
A little bit of salt has less of an effect than a lot of salt. So, if you add just a pinch of salt to a bucket of water, you may not observe much change in the freezing point, but if you add a few cups of salt to a bucket of water, you can expect a big change in the freezing point of water.
Can anyone think of an example of when lowering the freezing point of water might be useful? (Listen to student ideas.) What about on road surfaces during winter? Have you ever seen salt (or other anti-icing materials) sprayed on the roads? Why might people put salt on the roads? (Listen to student responses.) Putting salt on roadways is a way to make water remain liquid, and not freeze into slippery ice, even when the outside temperatures drop below zero degrees Celsius. When this happens, the roadways remain safer for driving since cars are much less likely to slip and go out of control with water on the roads compared to ice.
For what else could you use water that remains in liquid form below zero degrees Celsius? How about an instant freezer! If you have water at -10 degrees Celsius, you could cool down a bag of ice cream mix in the -10 degree Celsius water so it would freeze, thus making ice cream! To help you get a better understanding of these concepts, that is exactly what we are going to do and make today!
Before we start, plenty of other examples of engineering the freezing properties of liquids exist in our world, for example, antifreeze is used in vehicle engines to lower the freezing point of liquids that are needed to run cars and trucks (specifically, engine coolant), to keep the liquids from freezing into solids. During this activity, think about how you could apply the concepts you have just learned to cool materials engineering products that you use in your life!
Procedure
Before the Activity
- Gather materials and assemble one set for each group.
- Review the vocabulary and definitions, intended as a reference for teachers.
- Prepare a blank table on the classroom board (or an overhead projector), following the structure shown in Table 1.
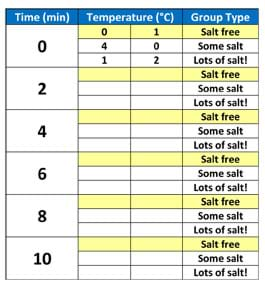
Example table for collecting groups' temperature measurements.
With the Students—Experiment Prep
- Conduct the Introduction/Motivation section that incorporates pre-activity call-out questions/answers (see the Assessment section).
- Divide the class into groups of four students each.
- Assign each group to be one of three types: 1) salt-free, 2) some salt, 3) and lots of salt.
- Assign team members to the following four roles: 1) temperature keeper, 2) timekeeper, 3) time and temperature recorder, and 4) prodder/stirrer.
- Provide each group with its set of materials.
- Review student roles within groups:
- The temperature keeper uses the temperature sensor, makes sure its temperature probe is within the water, and reads the temperature aloud for the recorder.
- The timekeeper uses a watch or wall clock to measure the passage of time, calls out the time at which to start, and calls out two-minute intervals.
- The time and temperature recorder uses pencil and paper to make a list with spaces for times and temperatures, and records the temperatures read by the temperature keeper when the timekeeper calls out two-minute intervals.
- The prodder/stirrer uses a wooden spoon to continuously keep the ice cream mix bag in motion within the bag of ice.
- Guide the groups to complete the following lab procedure, with slight variation on the addition of salt, depending on group type.
With the Students—Experiment Procedure

- Have each student write on his/her three Dixie cups: no salt, some salt, lots of salt!
- Fill the gallon-sized plastic bag about one-quarter full with ice. [prodder]
- Insert the temperature probe to the bottom of the bag of ice, and have the recorder write down both the start time and temperature [temperature keeper]
- Add different amounts of salt to the bag of ice, depending on group type: [prodder]
- No salt groups: Do not add any salt at this step; continue stirring
- Some salt groups: Add all of the quarter-cup salt baggie; continue stirring
- Lots of salt groups: Add all of the two cups salt baggie; continue stirring
- Call out the time every two minutes, with special notice for the 4-minute mark (when the ice cream mix baggie will be added). [timekeeper]
- After each two-minute interval is announced, read aloud the temperature reading on the temperature probe. [temperature keeper]
- Record the time as called by the timekeeper, then record the temperature as called out by the temperature keeper. [recorder]
- While students are measuring and recording data, compile example data from the three group types onto the table on the classroom board (or overhead projector). See the Assessment section for details.
- When the 4-minute mark is called by the timekeeper, place the ice cream mix baggie into the bag of ice. [prodder]
- Use the wooden spoon to keep the ice cream bag in motion near the bottom of the bag of ice. [prodder]
- After the 10-minute mark is called by the timekeeper, remove the ice cream mix baggie from the ice bag, and place it on a paper towel. [prodder]
- Have each person feel the bag on the paper towel and describe what the bag feels like Record five (or more) descriptive words. [recorder]
- Put spoonfuls of the three ice cream mixes exposed to different ice-salt environments into the three Dixie cups. [all]
- Have students use the plastic spoons to taste the ice creams formed under different conditions so they can understand the different freezing points created.
- Lead a class discussion and analysis session to compare results from the three group types, and draw conclusions. Expect students who bring their ice cream mixes to temperatures lower than 0 °C using salt-ice mixtures to get the icy, thick consistency they expect of ice cream, whereas students who use the salt-free ice water for cooling are unable to get their ice cream mixes to freeze, and see that their ice creams remain soupy liquids (albeit colder soupy liquids). See example questions in the Assessment section.
- Have students draw before/after set-ups of the experiment, labeling the temperatures on their diagrams.
- Have students reflect on the activity, an example of engineering the material properties of water for a specific application (to best freeze ice cream). Have them brainstorm other possible applications of this materials modification. See the Assessment section for details.
Vocabulary/Definitions
equilibrium: A stable state in which perturbation is required for a change to occur. For example, ice and water are in equilibrium at 0 °C, meaning that if both ice and water are at 0 °C, neither the amount of water nor the amount of ice will change unless a temperature change occurs.
freezing point: The temperature at which a material transitions between liquid and solid phases.
materials engineering: The intentional modification, manipulation and/or application of material properties with a purpose in mind.
mechatronic sensor: A type of electrical sensor that can measure temperature by converting an electrical reading to a digital display.
phase: A state of matter that a material exists in, such as liquid phase, solid phase or gaseous phase. Matter can exist in different phases depending on the environmental conditions of temperature, confinement volume and pressure.
Assessment
Pre-Activity Assessment
Call-Out Q&A Session: During the introduction, ask students a variety of questions relating to cooling limits (or freezing point) of water, heat transfer and how physical properties of materials can be modified. Refer to the Introduction/Motivation section and Investigating Questions section.
Activity Embedded Assessment
Real-Time Compilation of Group Temperatures: While students are actively engaged in measuring the water-salt-ice mixture temperatures and keeping the ice cream mixture in motion, go around the room to check on groups. Gather a sampling of the data being recorded (times and temperatures), and tabulate this information on the classroom board (or overhead projector) so it is available for the post-activity analysis and discussion. Seeing the data on the board gives students a real-time sense of the data that other teams are gathering.
Post-Activity Assessment
Eat Ice Cream: Have students taste and make observations of the three different cups of ice creams, each containing a spoonful from the three environmental set-ups. Tasting the ice creams that were formed under different conditions enables them to understand for themselves the different freezing points of the liquid waters.
Class Discussion and Analysis: While students are eating the three different ice creams, ask them a variety of questions relating to the experiment just performed. Write the questions and answers on the classroom board (or overhead projector). Groups that used salt-ice mixtures to cool their ice cream mixes to lower than 0 °C get the icy, thick consistency desired for ice cream; groups who used the salt-free ice water for cooling are unable to get their ice cream mixes to freeze and only get cold soupy liquids. Ask questions to cement the connection between colder cooling environment and the iciest ice cream.
Example questions:
- Which group type produced ice cream with a consistency closest to what you expect for ice cream? Why? (Answer: The "lots of salt" group.)
- Which salt composition helped us to generate the familiar consistency of ice-cream? (Answer: The ice baths with the highest amount of salt.)
- What kinds of salt conditions would you advise a person use if s/he wants to make soupy ice cream? (Answer: Low or no salt in the ice bath used to cool the ice cream mix.)
- What kinds of salt conditions should be used if you want to make ice cream with a hard consistency? (Answer: Lots of salt in the cooling ice bath.)
Diagram Drawing: Have students draw the before and after set-ups of their ice-water-salt-ice cream mixtures, and label the temperatures on their diagrams.
Engineering Reflection and Applications Brainstorming: Ask students to reflect on their experiences engineering the material properties of water for the application of ice cream freezing. Have them decide which salt concentration was the best for their applications, and discuss ways of making better ice cream in the future using this concept of materials modification. In small groups, have them brainstorm ideas for other ways that the type of materials modification completed in this activity could be applied in other settings. To get them started, remind them of familiar everyday examples, such as salted winter roads, de-icing airplanes and windshield wiper fluid that does not freeze.
Investigating Questions
How is the modification of material properties useful?
How cold can water get below zero, and still remain liquid?
What can be done to change the lowest temperature water can be while still remaining in a liquid phase?
Have you ever made ice cream using an old-fashioned ice cream maker with a hand crank? Did you use ice? Did you use salt? Describe the results — what was the consistency of the ice cream?
Safety Issues
Before the activity, check for students who have milk or dairy allergies.
Troubleshooting Tips
For the "lots of salt" groups, if the ice cream mix has not solidified after 5-10 minutes, add additional salt to the salt-ice-water solution.
If a LEGO Intelligent Brick with an attached LEGO temperature sensor is not available, use a simple thermometer instead for similar effect, albeit without the added perk of gaining familiarity with a mechatronic sensing system.
Activity Extensions
Graphing Practice: Have groups share their temperature data, calculating averages for each two-minute interval for each group type, and graph on one plot the data for the three group types.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to various types of energy with a focus on thermal energy and types of heat transfer as they are challenged to design a better travel thermos that is cost efficient, aesthetically pleasing and meets the design objective of keeping liquids hot.

Students learn the scientific concepts of temperature, heat and the transfer of heat through conduction, convection and radiation, which are illustrated by comparison to magical spells found in the Harry Potter books.

Students learn about the difference between temperature and thermal energy. They create thermometers using simple materials and develop their own scales for measuring temperature. They compare their thermometers to a commercial thermometer, and get a sense for why engineers need to understand the pr...
References
Learning Lesson: We All Scream for Ice Cream. Last modified January 5, 2010. JetStream - Online School for Weather, National Weather Service, NOAA. (activity inspiration; lower temperature limit of water given salt saturation) Accessed June 23, 2010. http://www.srh.noaa.gov/jetstream/ocean/ll_scream.htm
Make Ice Cream in a Bag. AllRecipes.com. Accessed June 22, 2010. (source of ice cream recipe) http://images.allrecipes.com/site/allrecipes/pdfs/icecream_in_a_bag.pdf
Copyright
© 2013 by Regents of the University of Colorado; original © 2009 Polytechnic Institute of New York UniversityContributors
Ursula Koniges; Leonarda Huertas; Donna Johnson; Ryan Caeti; Elina MamashevaSupporting Program
AMPS GK-12 Program, Polytechnic Institute of New York UniversityAcknowledgements
This activity was developed by the Applying Mechatronics to Promote Science (AMPS) Program funded by National Science Foundation GK-12 grant no. 0741714. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: March 30, 2022





User Comments & Tips