Summary
Students are introduced to various types of energy with a focus on thermal energy and types of heat transfer as they are challenged to design a better travel thermos that is cost efficient, aesthetically pleasing and meets the design objective of keeping liquids hot. They base their design decisions on material properties such thermal conductivity, cost and function. These engineering and science concepts are paired with student experiences to build an understanding of heat transfer as it plays a role in their day-to-day lives. While this introduction only shows the top-level concepts surrounding the mathematics associated with heat transfer; the skills become immediately useful as students apply what they know to solve an engineering challenge.
Engineering Connection
Energy is all around us and in everything we do. It is important for engineers to understand all the forms of energy: heat (thermal), light (radiant), motion (kinetic), electrical, chemical, nuclear and gravitational. This energy is typically either stored (internal) energy or working (kinetic) energy.
In our daily lives, we attempt to be energy conscientious or energy efficient for two main reasons: 1) to reduce cost associated with energy consumption, and 2) to minimize the environmental impact (reduce by-products of fuel production, minimize the consumption of raw materials used to produce energy, such as trees and oil). Thus, engineers consider energy in all their designs, whether from the cost associated with producing a plastic cup (raw materials, energy required to melt and form plastic into shapes, disposal of waste streams), or designing a cooling system for a computer (fan or liquid cooling) to help keep internal electronics from over-heating.
Learning Objectives
After this activity, students should be able to:
- Follow step-by-step procedures.
- Explain the role energy plays in their daily lives.
- Explain the basic modes of heat transfer and give examples of each.
- Apply an understanding of heat transfer to design and build a prototype thermos that is cost-effective, aesthetically pleasing, and meets the need of keeping a liquid hot over a long period of time.
Goals:
- Math competency: Skills and appreciation for data collection used in real life.
- Quantitative literacy: Ability to follow procedures and provide recommendations.
- Engineering applications: engineering design process, material selection and cost considerations.
- Cultural relevancy: Follow procedures, collaborative work in small groups and hands-on activities.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS3-3. Design, build, and refine a device that works within given constraints to convert one form of energy into another form of energy. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design, evaluate, and/or refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | At the macroscopic scale, energy manifests itself in multiple ways, such as in motion, sound, light, and thermal energy. Alignment agreement: Although energy cannot be destroyed, it can be converted to less useful forms—for example, to thermal energy in the surrounding environment.Alignment agreement: Criteria and constraints also include satisfying any requirements set by society, such as taking issues of risk mitigation into account, and they should be quantified to the extent possible and stated in such a way that one can tell if a given design meets them.Alignment agreement: | Energy cannot be created or destroyed—it only moves between one place and another place, between objects and/or fields, or between systems. Alignment agreement: Modern civilization depends on major technological systems. Engineers continuously modify these technological systems by applying scientific knowledge and engineering design practices to increase benefits while decreasing costs and risks.Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS3-4. Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics). (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. Alignment agreement: | Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems. Alignment agreement: Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down).Alignment agreement: Although energy cannot be destroyed, it can be converted to less useful forms—for example, to thermal energy in the surrounding environment.Alignment agreement: | When investigating or describing a system, the boundaries and initial conditions of the system need to be defined and their inputs and outputs analyzed and described using models. Alignment agreement: |
Common Core State Standards - Math
-
Graph linear and quadratic functions and show intercepts, maxima, and minima.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of engineering design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Washington - Math
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Graph linear and quadratic functions and show intercepts, maxima, and minima.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Washington - Science
-
Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Matter and Its Interactions
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- calculator
- thermometer
- 200 ml water
- Thermodynamics Worksheet, one per student
To share with the entire class:
- beaker tongs or hot gloves
- burner or hotplate
- 500-ml beaker
- 100-ml graduated cylinder
- assorted thermos construction materials (such as water, foil, cotton balls, paper cup, plastic cup, Styrofoam cup, sand, paint, foam insulation, masking tape, as listed on the worksheet)
- store-bought thermos, old or new, one or more different designs, to provide comparative testing data (make note of purchase price)
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/wsu_heat_activity] to print or download.Pre-Req Knowledge
Familiarity with collecting and plotting data, and the basic concepts for properties of matter.
Introduction/Motivation
We're going to be discovering the role energy, temperature and heat play in engineering and our daily lives. Specifically, you will apply what you know about heat transfer and energy to solve an engineering design challenge: You want to design a thermos that keeps hot liquids cold and that you can compare to the performance, aesthetic appeal and cost of a store-bought thermos. In order to effectively design the best thermos possible, we need to review some basic science concepts. You will apply these to solve an engineering challenge, just as an engineer would do!
Let's review some background information.
Energy
The two main forms of energy are 1) kinetic (or moving) energy, which can take the form of electrical, thermal or sound; and 2) potential (or stored) energy, which can be due to gravity or chemical bonds. The law of conservation of energy states that the total amount of energy in an isolated system remains constant. Energy cannot be created nor destroyed, but it can change forms. Energy likes to move until it is in equilibrium (or balance) with its surroundings. If a temperature difference exists in a system, then heat naturally moves from the higher temperature to the lower temperature. The greater the temperature difference between the high temperature and the low temperature, the greater the temperature gradient and the faster heat moves.
Temperature
The three main temperature scales are Fahrenheit, Celsius and Kelvin. But, dozens of types of devices, known as thermometers,exist and are able to measure temperature. Some thermometers are specially designed to measure body temperature, oven temperature, cooking meat temperature, and liquid/solid/gas temperature.
Fahrenheit is the classic system of measuring temperatures. Water freezes at 32 degrees Fahrenheit and boils at 212 °F. In the US, most temperatures are reported in Fahrenheit.
The Celsius scale is the modern system of measuring temperature and is typically used along with the metric system. The freezing point of water is 0 degrees Celsius, and the boiling point is 100 °C. Both Celsius and Fahrenheit are used when discussing our day-to-day weather temperatures. Celsius degrees are larger than Fahrenheit degrees.
The Kelvin scale is used in most science and engineering applications. The Kelvin scale is based on absolute zero, which is given the value of 0 degrees Kelvin. Water freezes at the value 273.15 °K and boils at 373.15 °K.
Below are the questions to convert between the three temperature scales:
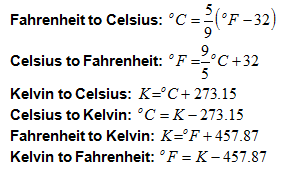
Thermodynamics
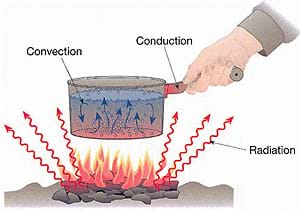
Thermodynamics is the study of heat and thermal energy, including how heat is transferred (or moved) between objects. The three main modes of heat transfer are conduction, convection and radiation. Through several examples we can see how we encounter each type of heat transfer on a daily basis.
Conduction is the transfer of heat through a solid object. When one part of an object is heated, the molecules within it begin to move faster and more vigorously; when these molecules hit other molecules within the object they cause heat to be transferred through the entire object.
If you hold an ice cream cone, the ice cream heats up because your hand is warmer than the ice cream. If you hold it long enough, you can observe the ice cream melt. The handle on a cast iron skillet gets hot as heat is transferred from the bottom where it contacts the oven burner.
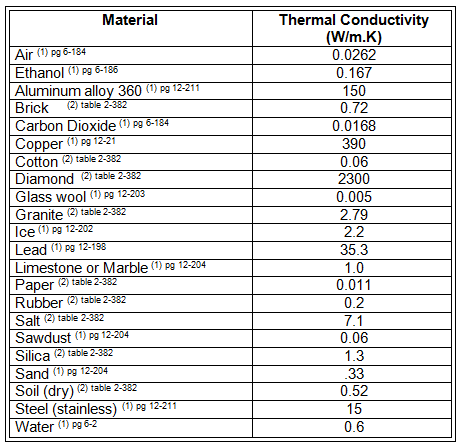
Some materials are better at conducting heat than others. Most good thermal conductors are metals, such as copper, silver, gold, iron, nickel, zinc and platinum. Many times, good thermal conductors are also good electrical conductors. Some poor thermal conductors are plastic, Styrofoam, air, water, rubber, cotton, silk, PVC, marble, wood, glass and mica. Thermal conductivity is the measurement of how "good" a thermal conductor a material is. The higher a materials' thermal conductivity, the better it is at conducting energy. The lower the thermal conductivity, the better a material is at being an insulator (prohibiting heat transfer).
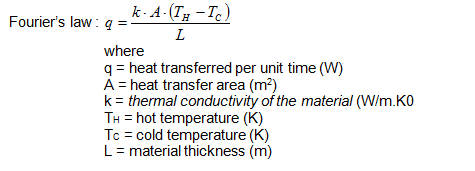
The mathematical relationship that describes heat transfer as a function of the material that heat is conducting through is known as Fourier's law and is given below. See Table 1 for the thermal conductivity of some common materials.
Convection is the transfer of heat by the movement of a fluid (water, air, etc.). In convection, it is important to remember that objects change position.
Warm air rises and cold air replaces it; which is why it is efficient to have a ceiling fan in a room with a fireplace. The hot air ends up near the ceiling so the fan helps move the warm to mix with the cooler air near the floor. If you hold your hand above the stove, you feel the heat as the hot air rises. Inside of a wall, air removes heat from a hot exterior wall then circulates to the colder interior wall where it loses the heat. You can see convection in a lava lamp, as the heated liquid moves upward and the cooler liquid circulates downward.
Radiation is the transfer of energy with no conductive medium (such as in space). That lack of medium means no matter exists for the heat to pass through (conduction or convection). Thus, radiation is a direct transfer of heat from one object to another, without heating the space in between. Radiation is the energy carried by electromagnetic waves (light), which might be radio waves, infrared, visible light, UV or gamma rays.
Background & Concepts for Teachers
Begin by introducing the concepts of energy, temperature heat and thermodynamics. Include ample opportunities for students to give their own examples of the types of heat transfer they encounter in everyday.
Review examples of the materials listed in Figure 1 to help the class relate to the materials while comparing thermal conductivities. If possible, provide actual examples that students can touch and feel, which is especially helpful for unfamiliar materials.
When discussing the mechanisms of heat transfer (conduction, convection, radiation), explain that most systems do not rely on only one type of heat transfer. Therefore, it is important not to omit any from the design of a system.
When discussing Fourier's law, have students look at the mathematical relationship and make a prediction of what will happen to the heat loss (q) if the thermal conductivity ( k ) is big? What happens to q if k is small?
Once students informally demonstrate understanding of the materials and concepts of heat transfer through class discussion, introduce the engineering design challenge: designing a thermos. Define "thermos" as applies to this challenge: a container used to hold a hot liquid with the goal that the liquid remain hot. As with all designed products, first a need and a purpose must be identified. For this activity, compare to the performance, aesthetic appeal and cost of an example store-bought coffee thermos.
Thermos engineering design challenge parameters:
- A (fictional) company hires the students (as engineering teams) to design a better thermos than what is already on the market.
- The winning prototype thermos must hold 100 ml water, allow the least heat loss over 10 minutes (starting from boiling water), and cost the least (cost per degree loss, $/°F). The heat loss must be less than the heat loss measured with the existing example product.
- Each group has a $3 budget for the purchase of materials.
- Any of the materials provided by teacher are available to be "purchased" for the design. (Permit students to bring in their own materials or the teacher can provide them for the class with the stipulation that students must "purchase" them from the teacher, staying under the $3 maximum spending limit.)
- Engineering teams may design and test as many different thermos designs as class time allows. Students can re-use materials from one design to the next, while keeping track of the individual cost of each design.
Procedure
Before the Activity
- Gather materials and make copies of the Thermodynamics Worksheet.
- Collect materials for students to use for their designs. See the list of example materials on the worksheet (water, foil, cotton balls, paper cup, plastic cup, Styrofoam cup, sand, paint, foam insulation, masking tape). If different/additional materials are provided, give students the thermal conductivity values and costs for those items, too.
- Following the procedures in the worksheet, test the store-bought thermos. Be sure to only test 100 ml water samples. Collect temperature data by measuring the temperature of the water inside the thermos. Start measurement from freshly boiled water and take a temperature reading every minute for 10 minutes. Add an extra twist by testing temperatures with and without a lid, to see the effect of a lid.
- Determine thermos cost (or purchase price works) and temperature loss in 10 minutes. Display this information on the classroom board for students to compare against their designs.
With the Students
- Divide the class into groups of two students each.
- Hand out the worksheets and have students assemble at lab stations with materials.
- Introduce any measurement tools students may be unfamiliar with, such as how to use and read values on a graduated cylinder, stopwatch, thermometer.
- Introduce the concepts of energy, temperature and thermodynamics, as described in the Background section.
- Describe the engineering design challenge and clearly outline the design requirements and constraints.
- Then direct students to proceed with the team design challenge, using the worksheet as a guide.
- Conclude by having teams share and compare their best thermos prototypes, reaching a conclusion on the winning design, the one that best meets the design requirements.
Vocabulary/Definitions
conduction: The transfer of heat through a solid object. When one part of an object is heated, the molecules within it begin to move faster and more vigorously; when these molecules hit other molecules within the object, they cause heat to be transferred through the entire object.
convection: The transfer of heat by the movement of a fluid (water, air, etc.).
heat transfer: The movement of heat from one body to another.
radiation: A direct transfer of heat from one object to another, without heating the air in between.
thermal conductivity: A property of a material that describes the materials ability to conduct heat.
thermal conductor: A material that allows for easy transfer of thermal energy. Large values of thermal conductivity. The opposite of a thermal insulator.
thermal insulator: A material that impedes (blocks) the transfer of thermal energy. Small values of thermal conductivity. The opposite of a thermal conductor.
thermodynamics: The study of heat and thermal energy, and how heat is transferred from one area to another.
Assessment
Worksheets: Have students use the Thermodynamics Worksheets to guide them through the design challenge activity. Review the data, graphs and answers on their completed worksheets to gauge their comprehension.
Safety Issues
- Remind students not to drink any chemicals, even those labeled as water or soda, because contamination is always possible.
- Alert students to practice safe lab practices especially when working with heat. Use tongs when handling hot containers and wear lab goggles to protect eyes.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the definition of heat as a form of energy and how it exists in everyday life. They learn about the three types of heat transfer—conduction, convection and radiation—as well as the connection between heat and insulation.

Students learn the scientific concepts of temperature, heat and the transfer of heat through conduction, convection and radiation, which are illustrated by comparison to magical spells found in the Harry Potter books.

With the help of simple, teacher-led demonstration activities, students learn the basic physics of heat transfer by means of conduction, convection and radiation. They also learn about examples of heating and cooling devices, from stove tops to car radiators, that they encounter in their homes, scho...

Students learn about the nature of thermal energy, temperature and how materials store thermal energy. They discuss the difference between conduction, convection and radiation of thermal energy, and complete activities in which they investigate the difference between temperature, thermal energy and ...
Copyright
© 2013 by Regents of the University of Colorado; original © 2009 Board of Regents, Washington State UniversityContributors
Courtney Herring (WSU Gene and Linda Voiland School of Chemical Engineering and Bioengineering)Supporting Program
CREAM GK-12 Program, Engineering Education Research Center, College of Engineering and Architecture, Washington State UniversityAcknowledgements
This content was developed by the Culturally Relevant Engineering Application in Mathematics (CREAM) Program in the Engineering Education Research Center, College of Engineering and Architecture at Washington State University under National Science Foundation GK-12 grant no. DGE 0538652. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: February 2, 2024







User Comments & Tips