Quick Look
Grade Level: 3 (3-5)
Time Required: 30 minutes
Expendable Cost/Group: US $2.00 This activity requires use of non-expendable (reusable) LEGO MINDSTORMS robot kit and temperature sensor (~$320); see the Materials List for details.
Group Size: 28
Activity Dependency: None
Subject Areas: Chemistry, Physical Science
NGSS Performance Expectations:

| 5-PS1-1 |
| 5-PS1-2 |
Summary
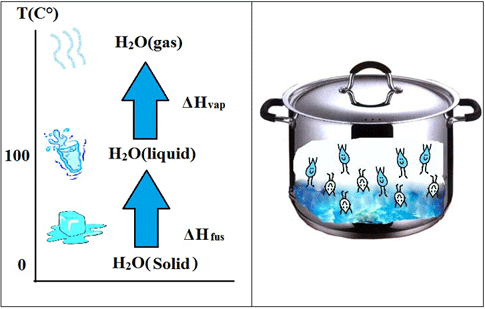
Students act as chemical engineers and use LEGO® MINDSTORMS® robotics to record temperatures and learn about the three states of matter. Properties of matter can be measured in various ways, including volume, mass, density and temperature. Students measure the temperature of water in its solid state (ice) as it is melted and then evaporated.Engineering Connection
Matter is all around us and found in just about any application in science, education, technology and engineering. When matter changes states, its physical properties also change. Chemical engineers are often interested in vapor liquid equilibrium data (the amount of species in equilibrium in a liquid and gas phase simultaneously). This type of data is found experimentally and is usually needed for product manufacturing and power plants. Mechanical engineers make measurements on solid materials such as composites or structural aspects in designs of airplanes and cars. Chemical engineers measure temperature and density in order to make everyday products such as toothpaste and medicines. All science and engineering fields deal with matter changing from one state to another.
Learning Objectives
After this activity, students should be able to:
- Describe the three states of matter.
- Explain that physical properties of matter can be measured (such as temperature, mass, volume, density, etc.).
- Explain the concept of evaporation.
- Identify the temperature at which water boils.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-PS1-1. Develop a model to describe that matter is made of particles too small to be seen. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to describe phenomena. Alignment agreement: | Matter of any type can be subdivided into particles that are too small to see, but even then the matter still exists and can be detected by other means. A model showing that gases are made from matter particles that are too small to see and are moving freely around in space can explain many observations, including the inflation and shape of a balloon and the effects of air on larger particles or objects. Alignment agreement: | Natural objects exist from the very small to the immensely large. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
5-PS1-2. Measure and graph quantities to provide evidence that regardless of the type of change that occurs when heating, cooling, or mixing substances, the total weight of matter is conserved. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Measure and graph quantities such as weight to address scientific and engineering questions and problems. Alignment agreement: | The amount (weight) of matter is conserved when it changes form, even in transitions in which it seems to vanish. Alignment agreement: No matter what reaction or change in properties occurs, the total weight of the substances does not change. (Boundary: Mass and weight are not distinguished at this grade level.)Alignment agreement: | Standard units are used to measure and describe physical quantities such as weight, time, temperature, and volume. Alignment agreement: Science assumes consistent patterns in natural systems.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
The process of experimentation, which is common in science, can also be used to solve technological problems.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
New York - Science
-
Develop a model to describe that matter is made of particles too small to be seen.
(Grade
5)
More Details
Do you agree with this alignment?
-
Measure and graph quantities to provide evidence that regardless of the type of change that occurs when heating, cooling, or mixing substances the total amount of matter is conserved.
(Grade
5)
More Details
Do you agree with this alignment?
Materials List
To share with the entire class:
- LEGO MINDSTORMS NXT Education Base Set (such as part #2095 for $159.98, available at https://shop.education.lego.com/legoed/education/NXT/NXT+Base+Set/5003402&isSimpleSearch=false; this set includes the 3 motors, wires and LEGO NXT brick needed for this activity)
- LEGO NXT temperature sensor ($27.96, available at https://shop.education.lego.com/legoed/education/NXT/NXT+Temperature+Sensor/9749&isSimpleSearch=false)
- ice cubes, 3 lbs (1.4 kg)
- metal pot
- hot plate or stove
- laptop computer on which to record/display data
- any cables required to connect NXT brick to computer
- States of Matter Pre-Assessment, one per student
- States of Matter Data Sheet, one per student
- States of Matter Post-Assessment, one per student
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/nyu_statesofmatter_activity1] to print or download.Pre-Req Knowledge
Familiarity with the three states of matter, physical properties of matter, using the five senses to identify matter, and measuring temperature.
Introduction/Motivation

Today, we will act as employees of a chemical engineering company and explore the various properties of water in different forms. Chemical engineers often deal with water (a liquid), ice (a solid) and water vapor (a gas) in their everyday work life. Most of our electricity comes from power plants that use the energy of steam (water vapor) to generate electricity. Steam is obtained by heating liquid water to very high temperatures to boil water.
As chemical engineers, we must know the temperature at which water boils and produces steam. Therefore, we will study how different forms of water behave under hot temperatures in order to discover the temperature at which water boils.
We will start with ice and observe how it behaves when exposed to a heat source (an electrical stove). Since we work for an engineering company, we would like to measure the temperature of our matter in an efficient way. We won't use a thermometer because thermometers cannot be used in a chemical plant where water is boiled in large quantities. In real-world industrial applications, thermometers are not used because that requires a person to manually take temperature readings. Imagine if your job was to sit a read a thermometer all day long? That doesn't sound like fun! Instead, temperature sensors are used and connected to computers and robots to automatically take temperature readings and record them. This is a far more efficient process.
For our activity, we wish to be safe and away from fire. Therefore, just like in a real chemical plant, we will use a robot to monitor the temperature and give us continuous temperature readings.
Today we will visually confirm the three states of water. We will start with water in its solid state (also known as ice), then apply heat to melt the ice. As the ice melts, our temperature-bot will monitor the temperature. What is your prediction? Will the temperature increase or decrease as ice is melted? We will continue melting the ice until it all melts and turns into liquid water. Then you will monitor the temperature as the liquid water begins to boil. How can you tell the water is boiling? You will be able to see the steam and recognize that steam is the third form of matter (gas).
Remember - our goal in this activity is to identify the boiling temperature of water from the temperature-bot reading in order for our steam power plant to operate successfully.
Procedure
Background
Students act as "chemical engineers" at a power plant facility that produces steam continuously by melting ice to water and then boiling liquid water to produce steam. The steam provides power to a motor, which in turn provides electricity for our homes. The engineering challenge for students: The engineers need to find out the temperature at which water boils and it is our responsibility to solve this problem by running an experiment. Students collect and analyze data from a temperature sensor that continuously displays temperature readings for them to closely monitor. Determining properties of matter plays a crucial role in industrial applications and these properties usually vary with different types of matter. For example, water and milk boil at different temperatures. It is important to pay close attention to these physical properties of matter since they are used in real-life applications.
Matter exists in three states: solid, liquid or gas. Students are introduced to matter by using their five senses to identify matter. They can also identify matter by its physical properties (such as shape, size, color, weight, etc.) except for certain types of gases. Students are shown that states of matter can be easily changed from solid to liquid to gas by melting ice on a stove, then by boiling water to change liquid to gas. A temperature sensor on a LEGO NXT robot enables students to determine the physical property of ice, liquid water, and water vapor, as well as identify the boiling point of water.
Before the Activity (for instructors)
- Gather materiails and make copies of the States of Matter Pre-Assessment, States of Matter Data Sheet and States of Matter Post-Assessment.
- Build a robot that incorporates a temperature sensor to monitor data. For instructions on assembling a simple robot, see: http://www.nxtprograms.com/NXT2/five_minute_bot/index.html. Be sure that the temperature sensor is attached to a motor and connected to the NXT brick.
- Make sure that all NXT brick battery packs are fully charged.
With the Students
- Give students 10 minutes to complete the pre-assessment handout. Then collect them.
- Discuss the experimental set-up and give students a rundown of the procedure.
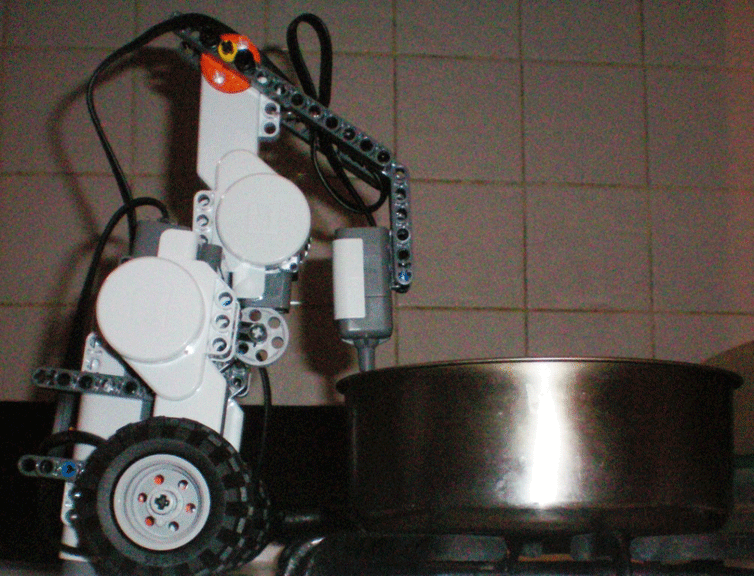
Experimental set-up to collect data with a temperature sensor and LEGO NXT robot.
- Review the pre-assessment with the entire class.
- Review with students the different states of matter and properties. What is the definition of a solid, a liquid, a gas? Review keywords and their definitions.
- Ask the students: How can the physical properties be measured in each state? (Solids and liquids can be weighed easily; gases cannot. Temperature can also be measured easily using temperature sensors. Physical dimensions can be measured for solid objects, such as ice cubes.)
- Introduce the temperature sensor and demonstrate how it will be used during the experiment. (A temperature sensor works similar to a thermometer but can give digital reading through the NXT once it is connected to the device. To demonstrate how the temperature sensor is used, download and run Temp_sensor_display.rbt on to the NXT brick.)
- Place the ice into a pot (about three pounds or 1.4 kg of ice)
- Identify the state of matter of ice. (solid)
- Place the pot on a hot plate or stove.
- Place the temperature sensor inside the pot. Then, turn on the heat.
- Turn on the NXT brick and use the NXT 2.0 data logging program to collect data.
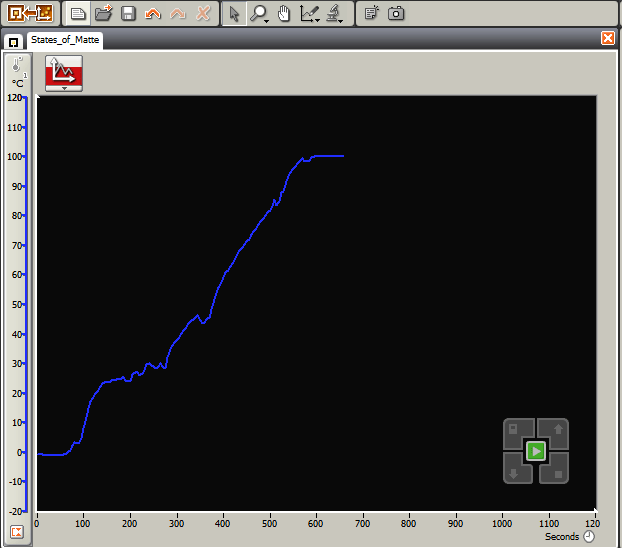
Data collection with temperature sensor of the three states of matter.
- The NXT data logging program automatically displays the data in a temperature vs. time graph on the computer, therefore no programming is required. The data logging software must be configured to the correct port that the temperature sensor is connected to in the NXT brick. Set the temperature unit to Celsius.
- Once the data logging software is running, the graph on the computer should give a temperature reading of 0° C.
- Once the NXT is continuously recording data, turn on the heat source.
- Once all the ice is melted, identify this state of matter. (liquid)
- Note that the temperature rises as the water is heating up.
- Once the water starts to boil and steam is observed rising, identify the state of matter of this water vapor. (gas)
- Record the temperature of the boiling point of water.
- The temperature of boiling water can be identified from the graph and stays constant at 100° C.
- After students record the correct boiling point of water (100° C) and identify the three states of matter (solid, liquid, gas), review the key activity point: You have now correctly identified the temperature at which water boils, which is necessary to know in order for the steam plant to operate.
Vocabulary/Definitions
condensation: The process of gas changing into liquid.
evaporation: The process of liquid changing into a gas.
freezing: The process of liquid changing into solid.
gas: Matter that has no definite shape or volume.
liquid: Matter that has a volume that stays same, but a shape that can change.
matter: Anything that takes up space.
melting: The process of solid changing into gas.
physical property: Anything that you can observe about matter by using one or more of your senses.
solid: Matter with a volume and shape that stays the same.
Assessment
Pre-Assessment:
Have students complete the States of Matter Pre-Assessment to evaluate their prior knowledge and understanding of states of matter and changes from one state to another.
Activity Embedded Assessment:
During the activity, have students complete the States of Matter Data Sheet to demonstrate their involvement in and understanding of the activity.
Post-Assessment:
Have students complete the States of Matter Post-Assessment to assess their understanding of the activity and the knowledge they gained about states of matter and changes from one state to another.
Safety Issues
While the water is being heated, the metal pot is extremely hot so make sure students keep away from the stove to avoid burns. Have them remain close to the laptop where the temperature is being logged.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to chemical engineering and learn about its many different applications. An associated hands-on activity gives students a chance to test their knowledge of the states of matter and how to make observations using their five senses: touch, smell, sound, sight and taste.

Students explore materials engineering by modifying the material properties of water. Using either a simple thermometer or a mechatronic temperature sensor, students learn about the lower temperature limit at which liquid water can exist—such that even if placed in contact with a material much colde...

Students learn about the difference between temperature and thermal energy. They create thermometers using simple materials and develop their own scales for measuring temperature. They compare their thermometers to a commercial thermometer, and get a sense for why engineers need to understand the pr...

Students are introduced to various types of energy with a focus on thermal energy and types of heat transfer as they are challenged to design a better travel thermos that is cost efficient, aesthetically pleasing and meets the design objective of keeping liquids hot.
References
Dictionary and Thesaurus® Miriam Webster Online. (definitions for gas, liquid, matter, solid) http://www.merriam-webster.com/dictionary/Matter
Bell, McLeod, DiSpezio, Frank, Krockover, Brink, Valenta, and Barry Van Deman. Science. Orlando: Harcourt School, 2008.
Copyright
© 2013 by Regents of the University of Colorado; original © 2010 Polytechnic Institute of New York UniversityContributors
Akim FaisalSupporting Program
AMPS GK-12 Program, Polytechnic Institute of New York UniversityAcknowledgements
This activity was developed by the Applying Mechatronics to Promote Science (AMPS) Program funded by National Science Foundation GK-12 grant no. 0741714. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: April 2, 2019






User Comments & Tips