Quick Look
Grade Level: 10 (9-11)
Time Required: 4 hours 30 minutes
(five 55-minute class periods)
Expendable Cost/Group: US $5.00
Group Size: 3
Activity Dependency: None
Subject Areas: Chemistry, Problem Solving
NGSS Performance Expectations:

| HS-PS1-6 |

Summary
Students create silver nanoparticles using a chemical process; however, since these particles are not observable to the naked eye, they use empirical evidence and reasoning to discover them. Students first look for evidence of a chemical reaction by mixing various solutions and observing any reactions that may occur. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. Students iterate on their initial process and test to see if they can improve the manufacturing process of silver nanoparticles.Engineering Connection
Chemical engineers develop processes to manufacture valuable chemicals through the application of chemistry, math, and engineering principles. Through these processes, they may improve upon existing processes or even create new, more efficient ones. In this activity, students take on the role of chemical engineers by building a valuable compound: silver nanoparticles. These nanoparticles have unique properties that are used in variety of modern applications such as in water treatment, medicine, and electronics. Students also apply the principles of chemistry and engineering to improve the existing manufacturing process.
Learning Objectives
After this activity, students should be able to:
- Describe the visible signs of a chemical reaction.
- Explain the principle of limiting reactants in chemical reactions.
- Explain why light scattering by nanoparticles produces unexpected colors.
- Refine a chemical process through experimentation.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Scientific inquiry is characterized by a common set of values that include: logical thinking, precision, open-mindedness, objectivity, skepticism, replicability of results, and honest and ethical reporting of findings.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-6. Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: In many situations, a dynamic and condition-dependent balance between a reaction and the reverse reaction determines the numbers of all types of molecules present.Alignment agreement: Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed.Alignment agreement: | Much of science deals with constructing explanations of how things change and how they remain stable. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Document trade-offs in the technology and engineering design process to produce the optimal design.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Arizona - Science
-
Predict the effect of various factors (e.g., temperature, concentration, pressure, catalyst) on the equilibrium state and on the rates of chemical reaction.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Texas - Science
-
organize, analyze, evaluate, make inferences, and predict trends from data; and
(Grades
10 -
12)
More Details
Do you agree with this alignment?
-
develop and use general rules regarding solubility through investigations with aqueous solutions;
(Grades
10 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- stereo microscope; if this isn’t available in a chemistry lab, check in with your biology department
- 3 disposable polystyrene petri dishes with lids, 100 mm x 15mm
- a piece of copper wire with no insulation, 4 cm (1.5 in) 16-24 gauge in width
- test tube rack
- pipette droppers
- 4 test tubes, 10 mm x 75 mm
- copy of Student Workbook, one per student
- permanent black marker
- deionized (DI) water, at least 1 L per group
- test tube brush
- 6 pieces of Glad Press’n Seal® Wrap, 2.5 cm2 (~1 in.2)
- safety goggles, one set per student
To share with the entire class:
- refrigerator (for storing the tanning solution)
- dish soap
- silver nitrate, 60 ml of 0.2 M solution
- Pu’erh tea to make 250 ml of tannin solution, 40% concentration tea, available in stores or on Amazon
- filter paper circles with a medium flow rate, available on Amazon
- sodium hydroxide, 50 ml of 0.1 M
- distilled vinegar, 50 ml
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/rice2-2264-silver-nanoparticles-nitrate-chemical-reaction] to print or download.Pre-Req Knowledge
- A familiarity with basic chemistry lab procedures and awareness of lab safety rules.
- A familiarity using and cleaning lab glassware such as test tubes.
- A basic understanding of the types of chemical reactions and signs of chemical reactions.
Note: this activity could also be used as an introduction to these topics.
Introduction/Motivation
Is it possible to turn base metals like copper or mercury into more valuable metals like silver or even gold? An ancient tradition called alchemy that was practiced extensively throughout the middle ages attempted to do just that. The aim of alchemy was to perfect certain objects by, for example, turning them into gold, or to create new substances that would help cure disease or even lead to immortality. While the practice of alchemy ended centuries ago, alchemists helped develop some of the laboratory techniques, theories, and methods that are used in basic scientific exploration, particularly in chemistry and medicine. While we cannot create gold out of thin air through chemistry, we are going to examine some fascinating processes that will allow us to create a type of silver that is used in a variety of modern applications.
(Follow along in the Student Workbook, 1.0 Lab: Introduction)
An atom of silver found in silver metal has an equal number of protons and electrons. Silver metal is metallic, shiny, and makes great jewelry. Silver ions, however, are missing one electron. Silver ions make up a different substance: silver nitrate. Unlike the related metal, silver nitrate is clear, brittle, and dissolves in water, which is why you never see silver nitrate jewelry.
Today we are going to give an electron to the silver ion in silver nitrate, and see if we can make silver metal again. When an ion gains an electron it is said to be reduced. When a substance loses an electron it is said to be oxidized. Chemistry research suggests that the element copper and tannin—an organic substance found in tea—may reduce silver nitrate by losing one of their electrons.
When substances are reduced and oxidized it is called a chemical reaction. Some signs of a chemical reaction include: unexpected temperature change, unexpected color change, formation of gas bubbles, or the formation of a new solid substance, also known as a precipitant. We will be looking for signs of a chemical reaction as evidence that we are making silver.
Note to teacher: The introduction is purposefully vague to allow students to discover that they are creating nanoparticles of silver. The goal is that students will discover empirical evidence before the explanation.
Procedure
Background
Silver nitrate (AgNO3) reacts with copper (Cu) to form copper(II) nitrate (Cu(NO3)2) and silver (Ag). This can be called a redox reaction because silver nitrate is reduced and copper is oxidized. This can also be called a single replacement reaction because copper replaces silver in the substance silver nitrate.
Silver nitrate and copper are the reactants and copper(II) nitrate and silver are the products. Silver is a precipitant because it is a new solid substance formed by a chemical reaction between soluble silver nitrate and copper. The balanced chemical reaction looks like:
2AgNO3 + Cu --> Cu(NO3)2 + 2Ag
The reaction continues until one of the reactants is completely consumed in the reaction. Once one of the reactants is gone, the reaction stops, leaving the other reactant. The reactant that runs out first is the limiting reactant.
Image 2 shows a magnification of the product of the chemical reaction between silver nitrate and copper. Silver is visible as precipitant dendrites on the copper wire. Copper(II) nitrate is visible as a green powder. Unreacted copper is also visible because it was not the limiting reactant.
The other reaction in the activity is between silver nitrate and tannins. Tannins are a class of many different large chemicals composed of carbon, hydrogen, and oxygen. One tannin is tannic acid, which has the formula C76H52O46. Tannins have many parts called phenols. Phenols can be oxidized and in return reduce silver nitrate. When tannins reduce silver nitrate they also produce nitric acid (HNO3). The balanced redox reaction looks like:
C76H52O46 + AgNO3 --> Ag + C76H51O46 + HNO3
Because tannins are large they block silver from growing like the dendrites on the copper wire. The particles of silver metal are so tiny, about 100 atoms, that you cannot see them. They are only a few nanometers across (a millionth of a millimeter) so we call them nanoparticles. The physical appearance of the solution of suspended silver nanoparticles appears brown.
Silver nanoparticles look brown because tiny particles scatter light. When light scatters, we see colors. Take cloud formations as an example. Water is not white, but we see white because the droplets of water scatter sunlight. We can also consider eye color. Blue eyes and green eyes do not have blue or green particles in them, but instead they have tiny particles of brown melanin. Melanin scatters light reflected in eyes. If there is some melanin in an eye, you see blue. If there is more melanin, you see green. When nanoparticles of silver scatter light, you see shades of yellow, amber, and brown.
Before the Activity
- Gather the materials for each group
- The plastic dishes can be the petri dish or its lid – both work in this activity.
- The actual length of the copper wire is unimportant, but should be at least 2.5 cm. (1 in.)
- Each student will need a copy of the Student Workbook.
- Set aside an area in the classroom where students can access and leave their plastic dishes for the duration of this five day activity.
- Create the tannin solution, silver nitrate solution, sodium hydroxide solution, and acetic acid solution before the activity. The tannin and silver nitrate solutions will be used on Day 1 and 4. The sodium hydroxide and acetic acid solutions will be used on Day 4.
- Directions for making the solutions:
- Tannin solution (brew tea)
- Rinse a 250 ml piece of glassware with deionized (DI) water
- Add 10 g of Pu’erh tea
- Fill the glassware with 150 ml of DI water
- Heat to a boil and then boil for 15 minutes (see Image 3)
- Filter about 100 ml of tea using a medium speed filter in a funnel into a clean 500 ml piece of glassware (see Image 4)
- Add 150 ml DI water to make a 40% concentration tea
- Label “tannin,” cover, and add dropper
- Keep refrigerated when not in use; tea will spoil if left out at room temperature


- Silver nitrate solution
- The molar mass of silver nitrate is 170 g/mol
- Rinse a 100 ml piece of glassware with DI water
- Add 2 g of silver nitrate to 60 ml of DI water
- Label 0.2 M silver nitrate, cover, and add dropper
- Sodium hydroxide solution
- The molar mass of sodium hydroxide is 44 g/mol
- Rinse a 100 ml piece of glassware with DI water
- Add 0.22 g sodium hydroxide and 50 ml of DI water
- Label 0.1 M sodium hydroxide, cover, and add dropper
- Acetic acid solution
- Rinse 100 ml piece of glassware with DI water
- Fill to 50 ml with distilled vinegar (not diluted)
- Label 0.8 M acetic acid, cover, and add dropper
With the Students
Day 1 (Student Workbook Sections 1.0 to 2.1)
- Start by organizing the students into groups and handing out a copy of the Student Workbook to each student.
- Present the Introduction/Motivation
- Introduce them to the Student Workbook by having them read 1.0 Lab: Introduction and fill in the missing definition blanks.
- Briefly cover Student Workbook, 1.1, 1.2, 1.3, and 1.4 with the students.
- Include extra lab safety practices related to your specific classroom situation.
- Note the location of all of the materials, and demonstrate how to label the test tubes and plastic dishes with a permanent marker.
- Note the location where you expect students to leave their plastic dishes overnight. Tell the students that moving the plastic dishes after the solutions are poured into them might mix and contaminate adjacent solutions.
- Describe how you want the test tubes cleaned and returned at the end of the lab.
- Tell the students to record their observations in the Student Workbook, 2.0 Worksheet: Observations and to answer the questions in the Student Workbook, 2.1 Worksheet: Questions. Students will work on the observations and questions together, with each individual recording their answers in their workbook. Students may complete unfinished questions as homework before the next class.
- Have students put on their goggles and follow the setup and procedure in the Student Workbook, 1.1, 1.2, and 1.3.
- A few minutes before the end of class, remind students it is time to finish the lab, and refer to Student Workbook, 1.4. Remind them to rinse their hands with tap water before they leave the classroom, and remind students that they need to complete Student Workbook, 2.1 Worksheet: Questions for homework.
Day 1 Tips For teachers
Initially the silver precipitant appears like a black coating on the copper wire. Once the dendrites grow longer, the coating takes on a fuzzy silver-gray appearance. The silver color is more obvious in brighter light.
Students may not notice that the solution in test tube A is turning green. It is subtle at low concentrations. Students might notice it if they compare it to the clear solution in test tube B.
If students do not notice the change in color in test tube D, have them compare it to test tube C. They started as the same color and noticing the color difference is evidence of a chemical reaction.
There is also a visual test for the presence of nanoparticles in solution. Using a laser pointer (if you have one on hand,) shine it through the side of one of the test tubes. Shining the laser through test tubes A, B, or C means you will not see the laser beam inside the solution. If it is test tube D, the presence of nanoparticles scatters the laser light, and you can see the laser beam inside the solution. This technique is similar to using chalk dust to see a laser beam in a classroom.
If students do not finish 25 minutes of observations, just make sure they notice a color change in test tube D and the precipitant in test tube A. The chemical reactions will continue to completion in the plastic dishes after they leave.
It not a problem if solutions touch and mix a little in the plastic dishes. Students will have lots of uncontaminated areas to examine on Day 2.
Remember, at the end of the day store the tannin solution in a refrigerator as they are prone to spoiling.
Silver nitrate solutions react in light to turn a surface black. Clean up spills with water and paper towels and avoid contact with skin. Students rinse their hands with tap water just in case their skin contacted silver nitrate.
It is important for the students to clean the test tubes. Cleaner glassware gives more accurate results.
Day 2 (Student Workbook Sections 3.0 to 5.4)
- Have students turn to Student Workbook 2.1 Worksheet: Questions and check for completion.
- Start the class by asking students which test tubes had chemical reactions and which ones did not. Ask them how they know there was or was not a chemical reaction. Ask them what they think was produced.
- Regardless of the answers, explain that today you will be examining the samples from test tubes A, B, C, and D that dried overnight for more evidence of what was produced.
- If this is the first time students are using stereo microscopes, demonstrate all of the following: how to adjust the magnification; how to adjust the eyepiece; how to use coarse and fine focus, how to place the sample to see different parts; how to turn on lamps above and below the sample.
- Have students collect their plastic dishes from Day 1.
- Have students follow the directions in Student Workbook 3.0 Lab: Stereo microscope. This section describes what each substance looks like through a stereo microscope, along with tips and photos.
- Have students complete Student Workbook, 4.0 Worksheet: Observations 2, describing what substances they see. There are also questions where students use their own words to describe what each substance looks like.
- Have students return their plastic dish samples and clean their work area.
- Assign students to read Student Workbook, 5.0, 5.1, 5.2, 5.3, and 5.4.
Day 2 Tips for Teachers
Student Workbook 4.0 is the end of the discovery part of the activity. At this point students have many pieces of empirical evidence that they produced silver nanoparticles in test tube D. The readings and particle diagrams in student workbook sections 5.0 to 5.4 explicitly describe what happened in each test tube and connects the students’ empirical observations to the chemical reactions.
The photos in Images 2, 5, and 6 are from actual student samples. The photos were taken with a cell phone camera through a focused stereo microscope eyepiece. You or your students could take similar photos to share or display.
Student workbook section 5.4 has a particle diagram that describes three reasons for a color change. This sets up the part of the lab where students will use differences in color as empirical evidence.
Save the dried plastic dish samples in case students want to compare what they produce on Day 4 to previous results.
The silver in D will be in a small sheet near the edge where the silver nanoparticles accumulate as the water evaporates. There will also be dendrites of pure silver throughout the dried sample. Each millimeter of a dendrite is 1 million silver nanoparticles in a line. There will be brown staining from the tannins and probably some unreacted silver nitrate crystals in the corners by the petri dish walls.
In test tube B, students get to see what unreacted silver nitrate crystals look like. This will help them when they see the same in other dried samples.
In test tube C, students get to see what unreacted tannin looks like. This will help them when tannin when they see the same in other dried samples.
Day 3 (Student Workbook Sections 6.0 to 8.0)
- Organize students into their groups and have them complete Student Workbook, 6.0 Worksheet: Questions 2. These reflective questions combine their empirical evidence with the informative readings.
- Check each group for understanding. Have students defend their answers by citing evidence. Ask questions like:
- How do you know that there was no reaction?
- What was your answer to question 9 on Day 1? Why did your answer change?
- What evidence is there that you produced silver?
- Why did it look brown?
- What would happen if I added more silver nitrate to …….? Why?
- After each group has defended at least one answer, transition to a whole-class discussion.
- Announce to the students that you are now finally convinced that they made silver nanoparticles in test tube D.
- Say to the students: “A chemical engineer would look at the manufacturing process you used to make silver nanoparticles and think of ways to make it better. If your job was to make silver nanoparticles, how would you want to make the manufacturing process better?”
- Listen to student answers. Afterwards, direct them to read Student Workbook, 7.0 Reading: Silver Nanoparticles 1, which describes some of the ways a chemical engineer might try to make the manufacturing process better. Direct them to read Student Workbook, 7.1 Reading: Silver Nanoparticles 2, which covers some of the chemistry principles a chemical engineer might use to change the manufacturing process.
- Inform the students that there will be a lab on Day 3. It will be just like Day 1 except they will use only two test tubes. The first will be a repeat of test tube D, the standard manufacturing process. The second will be unique to each group, a modified process with the goal of improving the manufacturing process in some way.
- Each student group needs to reach a consensus of how they want to change the manufacturing process. They also need to state how they believe the change will affect the amount, production speed, and size of the silver nanoparticles. Students also need to state if the change would affect the amount of silver nitrate lost in the production process.
- Each student needs to record their group consensus in Student Workbook, 10.0 Worksheet: Observations 3, as the answers to questions 23 and 24.
- Assign Student Workbook section 8.0 Worksheet: Complete Particle Diagrams for homework.
Day 3 Tips for Teachers
If students are unsure what change they should make, Student Workbook 7.1 lists a few options to choose.
Section 7.1 mentions a very simplified version of Le Chatelier’s principle. With nanoparticles the question of reversibility is complicated and a function of particle size and not just product concentration. In other words, the silver nanoparticle reaction is modeled as non-reversible and Le Chatelier’s principle is modeled as simply adjusting the forward reaction rate rather than the equilibrium point.
Generally, the slower the reaction rate, the smaller the silver nanoparticles. Extending the exposure time to hot or cold temperatures may change the silver nanoparticle size, with the added effect of the samples not having enough time to evaporate overnight.
Student Workbook 8.0 for homework allows students to use particle diagrams to guide them in their predictions of how changes in the manufacturing process will change the important factors. Every scenario is an exact copy of the particle diagram for test tube D in student workbook section 5.2 with one change applied.
Day 4 (Student Workbook Sections 9.0 to 10.0)
- Organize students into groups.
- Ask if any group wants to change their improved manufacturing process choice.
- Verify that the students completed Student Workbook 8.0.
- Have students follow the directions in Student Workbook, 9.0, 9.1, 9.2, 9.3, and 9.4. Tell them that it is similar to the lab on Day 1.
- Have students record their observations in Student Workbook, table 4, 10.0 Worksheet: Observations 3.
- A few minutes before the end of class, remind students where they need to leave their plastic dish, with samples D and E, overnight to dry.
Day 4 Tips for teachers
You can use the laser pointer from day 1 to prove students produced silver nanoparticles in both test tube D and E.
When students work on the Student Workbook, 8.0 particle diagrams they may realize that they want to change the manufacturing process in a different way to produce a better or different outcome.
Students are looking for differences between the original manufacturing process, test tube D, and the improved manufacturing process, test tube E. Students are mostly doing the same lab as day 1. They are looking for evidence that E is different from D. Today they will have color change and rate of color change. Tomorrow they will have evidence from the stereo microscope.
Day 5 (Workbook Section 10.1)
- Organize students into groups.
- Have students examine their dried samples with stereo microscopes.
- Direct students to complete Student Workbook, 10.1 Worksheet: Questions 3.
- Have students present their recommendation to the whole class, emphasizing improvements and tradeoffs.
- When they present, ask each group follow-up questions like:
- Why would producing more silver be beneficial?
- What evidence did you have that…?
- You saw ball like shapes in your sample. What could they be?
- You created smaller silver nanoparticles but you wasted a lot of tannin. Was it worth it?
- This other group suggested that it would be better to …. Do you agree?
- Collect the completed student workbooks.
Day 5 Tips for the Teacher
The stereo microscope tips listed in 3.0 are still valid.
There can be a lot of empirical evidence generated by comparing sample D and E.
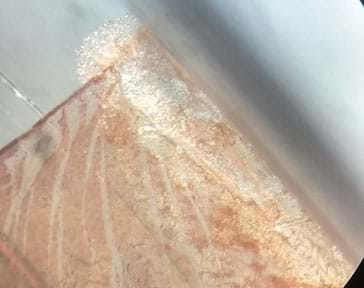

When comparing the two samples, both might contain a dark silver edge (as in Image 6), a lighter silver band near the edge, and silver dendrites elsewhere. However, one might have wider and denser silver features. Students may interpret that as a process producing more silver nanoparticles.
Students should combine the information from their observations of the chemical reaction in test tubes D and E, their analysis of the D and E dried samples, their previous experience analyzing samples, the information in the readings, and the information in the particle diagrams. Students have many sources to interpret change in silver nanoparticle production.
Without advanced equipment, analysis is very squishy and qualitative, but there are some general patterns. Slower reactions produce smaller silver nanoparticles (the best change). Lower temperatures, excess silver nitrate, and adding acid all slow down the reaction. Faster reactions produce larger silver nanoparticles. Higher temperatures, excess tannins, and adding sodium hydroxide increased the reaction rate.
Some of the evidence students may describe when answering the questions in Student Workbook, 10.1:
- Evidence of producing more silver metal:
- From the reading and prior knowledge: We added more silver nitrate and tannins so we created more silver.
- Test tube: The color is darker than expected.
- Dried sample: We see less silver nitrate in the corner - it all reacted so we produced more silver metal. We see a larger sheet of silver near the edges, so there were more nanoparticles. We see tighter packed and thicker dendrites.
- Evidence of producing less silver metal:
- From the reading and prior knowledge: We used less of one or both of the reactants.
- Test tube: The color is lighter than expected.
- Dried sample: There is more silver nitrate, so less reacted. The size of the silver sheet is smaller. Dendrites are thin and sparse.
- Evidence of producing silver metal faster:
- From the reading and prior knowledge: Hotter temperatures produce a faster reaction. Adding sodium hydroxide speeds up the reaction (Le Chatelier’s principle).
- Test tube: The color changed faster.
- Dried sample: A faster reaction produces larger nanoparticles. The sheet of silver is less smooth, grainier. There are fewer dendrites and more thick clumps of silver.
- Evidence of producing silver metal slower:
- From the reading and prior knowledge: Colder temperatures produce a slower reaction. Adding acetic acid slows down the reaction. Changing the reactant concentrations may change the reaction rate.
- Test tube: The color changed slower.
- Dried sample: A slower reaction produces smaller nanoparticles. The silver dendrites are finer.
- Evidence of wasting more silver nitrate:
- From the reading and prior knowledge: We added more silver nitrate and there already was excess silver nitrate. We added less tannin, so there would be excess silver nitrate.
- Test tube: Excess silver nitrate produces smaller particles. The color is lighter because the silver nanoparticles are smaller.
- Dried sample: We see a lot more silver nitrate on the edges and corners.
- Evidence of wasting less silver nitrate:
- From the reading and prior knowledge: I added excess tannin.
- Test tube: Excess tannin produces larger particles. The color is darker because the nanoparticles are larger.
- Dried sample: There is no visible silver nitrate. There might be a lot of visible tannin.
- Evidence of smaller nanoparticles
- From the reading and prior knowledge: The reaction is slower.
- Test tube: The color is lighter.
- Dried sample: The dendrites are thinner but the amount of silver is the same. The sheet of silver is smoother and thinner.
- Evidence of larger nanoparticles:
- From the reading and prior knowledge: The reaction is faster.
- Test tube: The color is darker.
- Dried sample: The sheet of silver is less smooth, grainier. There are fewer dendrites and more thick clumps of silver.
Vocabulary/Definitions
chemical reaction: A process that leads to the chemical transformation of one set of chemical substances to another. Oxidation is a type of chemical reaction.
dendrite: A characteristic, tree-like structure of crystals that grows when molten metal freezes.
Le Chatelier’s principle: Used to predict the effect of a change in conditions when a substance is subjected to a change in concentration, temperature, volume, or pressure; also known as the equilibrium law.
limiting reactant: A substance that is totally consumed when a chemical reaction is complete.
nanoparticle: Particles that measure between 1 and 100 nanometers (nm) in size.
oxidation : The loss of electrons during a reaction by a molecule, atom, or ion.
precipitant: A new solid substance formed from a solution.
product: The substances produced after a chemical reaction.
reactant: The substances that participate in a chemical reaction.
redox reaction: Short for a chemical reaction that includes reduction (red-) and oxidation (-ox) of reactants.
reduction: The gain of electrons during a reaction by a molecule, atom, or ion.
silver nanoparticle: Particles of silver that measure between 1 and 100 nanometers in size; composed largely of silver oxide.
silver nitrate: The substance AgNO3, composed of a silver ion and a nitrate ion.
single replacement reaction: A reaction by which one or more elements replace another element in a compound.
soluble: The ability for a substance to dissolve in water.
tannin: A class of astringent, organic molecules that bind to and precipitate proteins and various other organic compounds. One tannin is tannic acid which has the chemical formula C76H52O46.
Assessment
Activity Embedded Assessment
Student Workbook: Have students work through the Student Workbook. Sections 2.0, 2.1, 4.0, 6.0, 8.0, and 10.0 all provide opportunities for embedded assessment.
Post-Activity Assessment
Final Recommendation: Section 10.1 provides a framework where the students can act like a chemical engineer and present their final recommendation for improving the manufacturing process building silver nanoparticles.
Safety Issues
The activity requires glassware and dilute sodium hydroxide. Both require goggles and closed-toes shoes. Enforce that “nothing in this lab is safe to consume” because some people use silver nitrate and silver nanoparticles as nutrition supplements.
Troubleshooting Tips
The tannin solution is weak or not reacting well
Try using hotter water, steeping the tea for a longer period of time, or breaking the tea up into smaller pieces; using more tea while brewing also produces more tannins.
I want to eliminate the brown tint of the tea
Some substances that make the tea brown can be filtered out using vacuum filtration with micron filters – this may reduce the brown tint if you have access to them. Additional filtering with medium speed funnel filters provides no benefit.
Pu’erh tea actually is not required; any substance that is soluble and reduces silver works. Pu’erh tea just contains a lot of tannins. Tannins are a class of large molecules that have a lot of phenol groups. Phenol groups reduce silver and the large size of the tannins limit the size of the silver nanoparticles formed. You may use phenols extracted from fruit and leaves to produce the same results, which is referred to as “green” silver nanoparticle production. Other phenols may not have a brown tint.
Using more dilute tea also works but the reaction rate is slower. You would have to leave the solution in test tube D covered overnight.
There is a brown/black substance that covers the dried silver – how can I remove it so I can see the silver underneath?
The brown material from the tannins is not soluble in either ethanol or hot DI water. Mechanical removal will also remove the silver nanoparticles underneath.
Can I use a drying oven to speed things up?
Yes and no. The normal reaction takes hours. Using a drying oven soon after class will produce a lot less silver and leave a lot more silver nitrate because the reaction did not go to completion. Using a drying oven the next morning is a good idea if the sample are not completely dry.
I see threads in the samples
Those may be small airborne fibers that get caught in the sample; some might even get silver plated. To counter this effect, you can cover the petri dishes.
I want to change the concentration of the silver nitrate solution or tannins.
About 1 drop of 0.4M Silver nitrate reacts with about 1 drop of undiluted Pu’reh tea.
I want to use hydrochloric acid or sulfuric acid
Both 0.1M HCl and H2SO4 worked as well as distilled vinegar. HCl, H2SO4, and vinegar produce silver chloride, silver sulfate, and silver acetate respectively. All are partly soluble and reduce like silver nitrate.
When using sodium hydroxide I see light brown crystal nodules
That substance is silver hydroxide. In this case, the experiment may have had inadequate tannin concentration or chemical reaction time.
Activity Extensions
- Students could perform the inquiry section with a range of samples. Students could examine the effects of adding 0, 1, 2, 3, and 4 drops of excess tannin in five different samples.
- Students could collect all of the results from each group, and could then find patterns in multiple outcomes, refine the process to optimize the production of nanoparticles, and repeat the experiment with the refined process.
- Students could expand on the reduction of silver by metals other than copper, such as iron. Students could develop some of the activity series of metals. If a metal reduces silver nitrate it is more active than silver metal.
- Students could explore Mie scattering, Rayleigh scattering and Willis-Tyndall scattering. Students could make connections between their lab observations and light scattering principles and determine which type of scattering is occurring.
- Students could use redox titration to determine the strength of the tannins reduction potential. Dilute a sample of the tannins, add iodine and starch as indicators then titrate with hydrogen peroxide or some other oxidizing solution.
Activity Scaling
- For lower grades, students could create their own phenols for reducing silver. Some could boil red and green apple sand some plant leaves in water to see which extracts reduce silver. Use fruits and leaves that contain many flavonoids and antioxidants. The lab could consist of which fruits have the most antioxidants – they produce the most silver nanoparticles when mixed with silver nitrate.
- For higher grades, students can explore it as single replacement and redox reactions. They could balance the equations, use molarity, and apply stoichiometry to determine percent yield.
- For higher grades, students could mathematically apply Le Chatelier’s principle to predict changes in silver nanoparticle production before they attempt them.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Student teams conduct an experiment that uses gold nanoparticles as sensors of chemical agents to determine which of four sports drinks has the most electrolytes. Using some basic chemistry and physics principles, students develop a conceptual understanding of how gold nanoparticles function.
References
Loo, Yuet Ying et al. “Synthesis of Silver Nanoparticles by Using Tea Leaf Extract from Camellia Sinensis.” International Journal of Nanomedicine 7 (2012): 4263–4267. PMC. Web. 6 Jan. 2018.
Tippayawat, Patcharaporn et al. “Green Synthesis of Silver Nanoparticles in Aloe Vera Plant Extract Prepared by a Hydrothermal Method and Their Synergistic Antibacterial Activity.” Ed. Maria Rosaria Corbo. PeerJ 4 (2016): e2589. PMC. Web. 6 Jan. 2018.
Zainal Abidin Ali, Rosiyah Yahya, Shamala Devi Sekaran, and R. Puteh, “Green Synthesis of Silver Nanoparticles Using Apple Extract and Its Antibacterial Properties,” Advances in Materials Science and Engineering, vol. 2016, Article ID 4102196, 6 pages, 2016. doi:10.1155/2016/4102196
Copyright
© 2019 by Regents of the University of Colorado; original © 2017 Rice University ERC-RETContributors
Richard DainesSupporting Program
Engineering Research Center for Nanotechnology Enabled Water Treatment Systems (NEWT) RET, Rice UniversityAcknowledgements
This curriculum was based upon work supported by the National Science Foundation under Rice University Engineering Research Center for Nanotechnology Enabled Water Treatment Systems (NEWT) RET grant no.1449500. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Special thanks to Dr. Shahnawaz Sinha, Dr. Paul Westerhoff, and Dr. Francois Perreault at Arizona State University for the introduction to the uses and synthesis of silver nanoparticles. Special thanks to Christina Crawford at Rice University for encouraging writing this activity.
Last modified: August 28, 2019



User Comments & Tips