Quick Look
Grade Level: 8 (9-10)
Time Required: 45 minutes
Expendable Cost/Group: US $0.00 The cost per group is around $20.
Group Size: 2
Activity Dependency: None
Subject Areas: Biology, Chemistry, Life Science
NGSS Performance Expectations:

| MS-PS1-2 |

Summary
Students take on the role of chemical engineers to find a safe way to remove surface scalants like calcium carbonate that is both effective and safe. With teacher guidance, students learn about the chemistry involved as they work to remove scaling in a pipe using natural substances. Along with exploring designs based in chemistry, they consider the safety and environmental impacts of their solutions.Engineering Connection
Chemical engineers use principles of chemistry, biology and physics to transform processes, energy and materials. Some processes can be performed with natural materials and resources. However, others require strong and hazardous materials. Chemical engineers take a lot of time to consider the safety and environmental impacts of their solutions.
Learning Objectives
After this activity, students should be able to:
- Describe removal techniques for calcium buildup in a pipe.
- Outline a scientific and engineering design.
- Calculate percent change.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze and interpret data to determine similarities and differences in findings. Alignment agreement: Science knowledge is based upon logical and conceptual connections between evidence and explanations.Alignment agreement: | Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Alignment agreement: Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.Alignment agreement: | |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Attend to precision.
(Grades
K -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Texas - Math
-
apply mathematics to problems arising in everyday life, society, and the workplace;
(Grade
8)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- galvanized pipe, ~19 mm diameter and 15 cm long, with scaling already present; (prepare this prior to giving the pipes to the students; see Before the Activity)
- Removing Scaling Student Worksheet (1 per student)
For the entire class to share:
- 5 lemons
- 5 limes
- 5 oranges
- 5 grapefruit
- pipettes (500 in a box)
- juicing tool
- lab goggles (enough for the class)
- 3 cutting boards
- knives
- digital scale
- 10 mL spray bottles, 20 total
- pH paper (5 boxes)
- 250 mL Beakers, 20 total
- powdered citric acid bag (~600 mL bag; optional)
To prepare the scaled pipe:
- powdered calcium carbonate (~600 mL bag)
- water
- small bowl and mixing apparatus
- oven
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/rice2-2535-removing-scaling-sources-citric-acid] to print or download.Pre-Req Knowledge
A basic understanding of chemistry and elements.
Introduction/Motivation
(Have the Removing Scaling Using Citric Acid Presentation ready to show the class; show the following video, a Drano advertisement from 1993: https://www.youtube.com/watch?v=LvujMpLfKuA.)
What problem is being presented in the video? What is the drain cleaner doing to the pipes to help solve that problem? While drain cleaners are an effective way to clean pipes, these products are caustic and toxic, and extremely harmful to humans.
That said, is there an alternative solution, so we can keep the pipes in our homes and municipal systems clean, without having to resort to caustic chemicals? In order to successfully clean the pipes of our water treatment plants and homes, it is important to find a safer option. (Watch the following video on the uses of citric acid around the home, “Lemon Tips: 8 Great Household Uses for Lemons” - https://www.youtube.com/watch?v=HaRtVw-uN_Q. What kind of conclusions can we make about citric acid? Why might it be effective as a safe cleaner?
Now that we have a little background, it’s our turn to take on the role of chemical engineers! Let’s work on developing and testing a product to remove the scaling using a wonderful natural source: citrus fruit!
Procedure
Background
Limescale or scalant is a hard chalky deposit, consisting mainly of calcium carbonate (CaCO3), that builds up inside kettles, hot water boilers, and pipework. This buildup also occurs on the inner surfaces of old pipes and other surfaces where "hard water" has evaporated.
The type found deposited on the heating elements of water heaters consists mainly of calcium carbonate (CaCO3). Hard water contains calcium (and often magnesium) bicarbonate or similar ions.
When calcium carbonate reacts with water that has dissolved carbon dioxide, it forms a calcium bicarbonate. As new cold water with dissolved calcium carbonate is added and heated, the process continues. In this process, the CO2 gas is removed repeatedly, and the carbonate concentration increases, and thus more calcium carbonate precipitates. At this point, it is practically insoluble—that is, unless it is exposed to an acid. The calcium carbonate dissolves in the acid and when it does so it produces carbon dioxide.
Citric acid can cause this reaction. It is more acidic than carbonic acid, and can react with carbonates to release carbon dioxide and water (CO2 + H2O). Citric acid also can form citrate salts when it undergoes acid-base reactions with other salts. Citric acid is a potential solution to the described buildup in pipes.
In this activity, students can use the following formula to calculate a percent change of mass to determine if they were successful in removing scaling from the pipe.
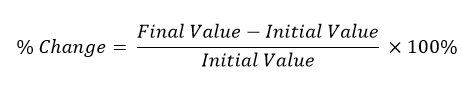
Before the Activity
- Gather materials and make copies of the worksheet.
- Follow the directions to create homemade scaled pipes (to be done no less than one week before beginning the lab):
- Mix a 2:1 ratio of calcium carbonate (CaCO3), to water in a bowl until the texture is similar to a paste. Spread the paste on the inside of the pipes. Put the pipes in the oven at 204°C (400°F) for 15 minutes. Do another coat for more buildup on the inside of the pipe. Aim to have at least a 12 mm (0.5 in) layer of buildup (the thicker the layer, the better).
With the Students
- Distribute copies of the worksheet. Begin the Removing Scaling Using Citric Acid Presentation and show the video on slides 2 and 4. Students reflect on their own knowledge and the issue of safely removing scaling.
- Divide class into groups of two students.
- Show slide 5. Bring the class attention to the table in the front of the room that has the supplies laid out. Explain that each partner pair must choose only one of the given materials to clean the scaling from their pipe.

- Set up a timer for 5 minutes with guiding questions to help the students brainstorm and create their protocol that they follow as a partner pair. Students consider length of application, pH of solution used, and how the solution will be applied to their surface.
- Have each partner take the initial mass of their pipe and write the value sown on their lab sheet in the corresponding box.
- Give students 10 minutes to test their method. Remind students to take the final mass of their pipe after they complete their protocol.

- Using the initial mass and the final mass of their pipe, each partner pair comes up with a percent change to see if they were successful in removing the scaling on their pipes. (Typically the rate of change won't be over 5% because of how light the citric acid is. Expect student rates of about 2-3%.) Students determine if they did remove scaling from their pipe using their specific method.
- Optional: have students test another design method using a 2:1 ratio of citric acid powder to water; use this solution to remove excess calcium carbonate from the pipe. Ask students why this method might work or not based on their experience using citrus fruit.
Vocabulary/Definitions
calcium carbonate: A chemical compound with the formula CaCO₃. It is a common substance found in rocks as the minerals calcite and aragonite and is the main component of pearls and the shells of marine organisms, snails, and eggs.
citric acid: A weak organic acid that has the chemical formula C₆H₈O₇. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
percent change: In quantitative science, the difference used to compare two quantities while taking into account the "sizes" of the things being compared. The comparison is expressed as a ratio and is a unitless number.
scaling/biofouling: The accumulation of microorganisms, plants, algae, or small animals on wetted surfaces that have a mechanical function, causing structural or other functional deficiencies
Assessment
Pre-Activity Assessment
Prior Knowledge: Before showing the video on slide 4 of the Removing Scaling Using Citric Acid Presentation, ask students what they know about the juice that can be found in citrus fruits in order to assess what students already know about citric acid.
Activity Embedded (Formative) Assessment
Worksheet: Ensure students complete the Removing Scaling Student Worksheet as they work through this activity.
Post-Activity Assessment
Percent Change: At the end of the lab, students calculate their percent change. Using this, students determine if they were successful at removing scaling or not.
Safety Issues
- Use eye protection (goggles) when handling any citric acid.
- Have the teacher cut the fruits so students don’t have to handle the knives.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the fundamental concepts important to fluid power, which includes both pneumatic (gas) and hydraulic (liquid) systems.
Copyright
© 2020 by Regents of the University of Colorado; original © 2020 Rice UniversityContributors
Kaitlin Bartuska; Tanya Rogers; Christina CrawfordSupporting Program
Research Experience for Teachers (RET), NEWT (Nanotechnology Enabled Water Treatment), Rice UniversityAcknowledgements
This curriculum was based upon work supported by the National Science Foundation under Rice University Engineering Research Center for Nanotechnology Enabled Water Treatment Systems (NEWT) RET grant no.1449500. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Last modified: October 31, 2020



User Comments & Tips