Quick Look
Grade Level: 4 (3-5)
Time Required: 1 hours 45 minutes
(can be split into two 50-minute sessions)
Expendable Cost/Group: US $3.00
Group Size: 3
Activity Dependency: None
Subject Areas: Data Analysis and Probability, Earth and Space, Measurement, Physical Science, Science and Technology
NGSS Performance Expectations:

| 4-PS3-4 |

Summary
To explore different ways of using solar energy, students build a model solar water heater and determine how much it can heat water in a given amount of time. Solar water heaters work by solar radiation and convection.Engineering Connection
Engineers use the principles of radiation and convection in many applications. For example, in the engine cooling system of a car or motorcycle, pumps move a fluid (antifreeze or engine coolant) through the engine. As this fluid flows throughout the engine, it absorbs the heat produced by the motor (convective and conductive heat transfer), which cools the engine. The primary purpose of the coolant is to transfer heat to cool the engine — just the opposite of the purpose of a solar water heater, which is to transfer heat to warm the house.
Learning Objectives
After this activity, students should be able to:
- Build a basic solar water heater.
- Explain how the sun can be used to heat water.
- Identify the parameters engineers can modify to improve solar water heaters.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
4-PS3-4. Apply scientific ideas to design, test, and refine a device that converts energy from one form to another. (Grade 4) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Apply scientific ideas to solve design problems. Alignment agreement: Analyze and interpret data to make sense of phenomena using logical reasoning.Alignment agreement: Develop a model to describe phenomena.Alignment agreement: | Energy can also be transferred from place to place by electric currents, which can then be used locally to produce motion, sound, heat, or light. The currents may have been produced to begin with by transforming the energy of motion into electrical energy. Alignment agreement: The expression "produce energy" typically refers to the conversion of stored energy into a desired form for practical use.Alignment agreement: Possible solutions to a problem are limited by available materials and resources (constraints). The success of a designed solution is determined by considering the desired features of a solution (criteria). Different proposals for solutions can be compared on the basis of how well each one meets the specified criteria for success or how well each takes the constraints into account.Alignment agreement: | Energy can be transferred in various ways and between objects. Alignment agreement: Engineers improve existing technologies or develop new ones.Alignment agreement: Science affects everyday life.Alignment agreement: |
Common Core State Standards - Math
-
Represent and interpret data.
(Grade
4)
More Details
Do you agree with this alignment?
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
-
Graph points on the coordinate plane to solve real-world and mathematical problems.
(Grade
5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Models are used to communicate and test design ideas and processes.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Energy comes in different forms.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Visual displays are used to interpret data.
(Grade
5)
More Details
Do you agree with this alignment?
-
Graph points on the coordinate plane to solve real-world and mathematical problems.
(Grade
5)
More Details
Do you agree with this alignment?
Colorado - Science
-
Identify and describe the variety of energy sources
(Grade
4)
More Details
Do you agree with this alignment?
-
Describe the energy transformation that takes place in electrical circuits where light, heat, sound, and magnetic effects are produced
(Grade
4)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 30 cm of aquarium tubing
- Cardboard toilet paper tube (ask students to bring from home)
- Aluminum foil (size = length of paper tube x ½ its diameter)
- 1 self-adhesive fastener for binding holed paper, to hold the tubing in place (available at office supply stores) (alternative materials could be used, such as hot glue, or small cut and glued pieces of corrugated cardboard or plastic comb binding)
- 2 brass brad fasteners (or staples or glue)
- 1 Styrofoam cup
- Scissors
- Ruler (for measuring cardboard tube and foil)
- Solar Water Heater Worksheet, one per student
At each light station:
- 1 gooseneck lamp (using a 100 W bulb)
- 1 measuring cup (or jar with volumes marked, beaker or graduated cylinder)
- 1 small jar for collecting the water (or use another Styrofoam cup)
- A thick book and a thin book (to make step-like stands)
- A non-mercury thermometer
For the entire class to share:
- Black paint
- Sunglasses (optional; one for each student if the activity is done outside; at least one per team if using a lamp; ask the students to bring their own)
- Modeling clay (optional; may help to keep the tubing poked in the cup from leaking)
- Protective table covering
Reuse/recycling/disposal note: Recycle the cardboard toilet paper tubes, removing the self-adhesive fastener first. Recycle or reuse the aluminum foil. Fold the foil flat (not into a ball) if recycling. Save (and reuse) the tubing, brad fasteners, Styrofoam cups and jars.
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_energy2_lesson09_activity2] to print or download.Introduction/Motivation
We can use the energy from the sun — a renewable energy source — for many things. We can use it to heat and light our homes, dry our clothes, cook our food, but how about to heat our water? Have you ever been in a shallow swimming pool or walked through a shallow puddle on a hot day? Sometimes the water in shallow pool or puddle warms from the sun. This is an example of the transfer of solar energy into heated water. So, yes, solar energy can be used to heat water.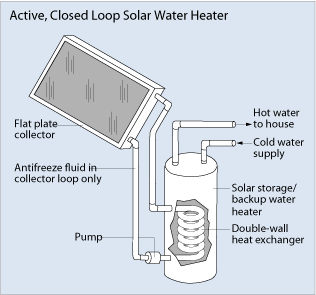
Most U.S. homes have some sort of water heater appliance that heats the water supplied to sinks and showers. Sometimes the hot water is also used to heat the house. Engineers have developed water heaters powered by gas, electric and solar. Gas water heaters burn natural gas (or other fossil fuels) to heat the water inside a storage tank. Electric water heaters use a heating element (like you may have seen inside your electric oven, an electric stovetop burner, or your toaster) in the storage tank, which transfers heat to the water. In solar water heaters, solar energy warms the water. Electric water heating systems use the most energy to produce the same amount of heat. The most efficient and reliable water heating systems use a solar water heater to provide most of the heating, with a gas water heater to supply any extra heating required. Do you know how the water is heated in your home?
Like any other solar energy system, solar water heating can be active or passive. Passive solar heating is when solar energy is used to heat something without requiring electrical or mechanical components. Active solar methods use electrical or mechanical pumps to make fluids flow through the collectors (see Figure 1). Active solar water heaters are more efficient than passive solar water heaters, but they require more equipment and engineering expertise to build and install, and are more expensive to run.
Passive solar water heaters are what we are going to look at today. Solar water heaters work by solar radiation and convection. Most passive solar heaters have one or more dark-colored storage tanks. These tanks are located inside an insulated box with glass or plastic on the side facing the sun. Can you think why? Well, the dark storage tank absorbs the heat radiating from the sun and the insulation keeps the heat from escaping from the water. Cold water flows in at the bottom of the tank and hot water flows out at the top to a water heater, where it can be heated more, if necessary. During winter, these outdoor solar water heaters are either drained or protected from freezing temperatures.
To make a solar water heater the right size for a household, engineers figure out how much water they have to heat. Think about it, how much hot water do you use in a day? Hot water is used to cook, clean and to wash dishes, clothes and ourselves. Solar water heaters can be less expensive than other water heaters, as long as you have a place to store the hot water when the sun goes down.
Today, we are going to be engineers and build a model solar water heater. Let's see how much solar energy it takes to heat up a little bit of water and then think about how we might design a solar water heater for our home or our school.
Procedure
Water Heater Background
Active solar heaters use pumps to circulate fluids through the collectors. In areas without long freezing periods or hard or acidic water, the water can be heated directly. These systems are known as direct or open-loop systems. Otherwise, a heat-transfer fluid — such as propylene glycol, Freon or distilled water — is pumped through the collector. Heat is transferred from the warmed fluid to water through heat exchangers placed in the tank. Active systems that use a heat-transfer fluid are called indirect or closed-loop systems. Active solar water heaters are more efficient than passive solar water heaters, but they require more equipment and engineering expertise to build and install, and are more expensive to run.
Passive solar water heaters can be batch systems or thermosiphon systems. Batch systems (or integral collector storage systems) have one or more dark storage tanks. These tanks are inside an insulated box with glass or plastic on the side facing the sun. Cold water flows in at the bottom and hot water flows out at the top to a conventional water heater, where it can be heated more, if necessary. The system is operated by the water pressure from city mains or a well. During winter, batch solar water heaters need to be drained or protected from freezing temperatures. Thermosiphon solar water heaters have a storage tank located above the collector. As water is heated in the collector, it rises up to the storage tank. Cooler water flows down pipes to the bottom of the collector and circulates through the system again. Passive systems can be more reliable than active systems because there are no electrical components to maintain.
The storage tanks for solar hot water systems are similar to other water heaters, but usually hold about 80 gallons. These tanks are highly insulated to prevent heat loss from the tank to the surroundings. Because solar water heaters can heat water to about 180ºF (82ºC), storage tanks have mixing valves so cold water can be added to the hot water from the tank. Mixing valves are usually set to about 120ºF (49ºC) to prevent burning.
Before the Activity
- A few days in advance, cut the aquarium tubing into 30 cm lengths.
- Divide the class into teams of three or four students each. Have student teams paint their piece of tubing black. Let it dry on a sheet of newspaper.
- If performing the activity indoors, prepare a few light stations that each contain: gooseneck lamp with 100 W bulb, thick book, thin book, jar for measuring water, and jar for collecting water.
- Gather materials and make copies of the Solar Water Heater Worksheet.
With the Students — Water Heater Preparation
- Paint the aquarium tubing black.
- Measure the length and circumference of the toilet paper tube. Record these measurements.
- Make the parabolic solar collector by first cutting the toilet paper tube in half lengthwise.
- Place the two halves of the tube back to back (see Figure 2). Attach them together at both ends with the brass brads (glue or staples also work).
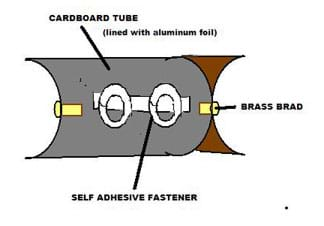
- Cut a piece of aluminum foil large enough to cover the inside surface of one of the tube halves. The foil dimensions should be the length of the tube by one-half of the circumference of the tube.
- Line the inside of the tube with the aluminum foil, keeping the shiny facing up.
- To secure the tubing along one trough of the toilet paper tube, make and attach a fastener of some sort to provide loops to hold the tubing (or, instead, use hot glue at a later step). Possible ways to do this: Use a pencil to bend the metal fastener prongs into circles, and stick the self-adhesive fastener lengthwise in the middle of one of the tube halves. Bend the prongs straight up. Measure the position of where you want to insert the prongs in the cardboard tube, and use these measurements to make slits in the foil for the prongs. Later, the tubing will be secured by sliding it through these loops. Or, attach short pieces of a comb binding, or ¼-inch thick pieces of cardboard, etc. Make sure the fasteners do not block light from falling directly on the tubing.
- Measure about ¼ inch (64 mm) up from the bottom of the Styrofoam cup and make a mark. Use a pencil to poke a small hole in the side of the cup. Be careful not to make the hole bigger than the pencil.
- Insert one end of the tubing into the hole. It should fit snugly. Use clay around the joint, if desired.
With the Students — At the Light Stations
- Arrange the components into a model solar water heater, referring to Figure 3.
- Place the cup on a thick book. Place a thinner book in front of the first book.
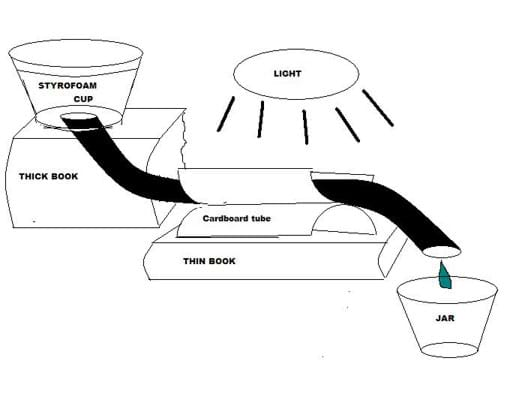
- Secure the tubing along the top trough of the parabolic collector made from the toilet paper tube. Use hot glue, or carefully slide the free end of the tubing through the parabolic collector fastener loops made and attached earlier. Place the parabolic collector on the thinner book so it is slightly lower than the Styrofoam cup.
- Place a small jar under the free (bottom) end of the tubing. The jar should be on the lowest level. Check each team's solar water heater setup before continuing.
- Angle the gooseneck lamp so it is about 3 cm from the parabolic collector. Check that each team's setup looks like Figure 3 before continuing.
- Have one student measure 100 ml of water and record the temperature of the water.
- The student with the water pours it into the Styrofoam cup. Another student immediately adjusts the height of the collecting jar so the water runs out very slowly (by raising the height of the jar so the tube is horizontal).
- A third student takes temperature readings of the water in the collecting jar every 30 seconds, recording the temperatures on the worksheet. This student should circle on their worksheet data chart the final temperature reading when the last of the water drained into the collecting jar. Do not pour this water down the drain!
- The team members change roles for round two. One student pours the water from the collecting jar into a measuring cup (or jar) and hands it to a second student who pours the water back into the Styrofoam cup. Another student immediately adjusts the height of the collecting jar so the water runs out very slowly. A third student takes collecting jar temperature readings every 30 seconds, again circling on the worksheet data chart the final temperature reading when the last of the water drained into the collecting jar.
- Repeat this process until the students have taken measurements for three or four rounds.
- Have students complete the worksheet questions and graph their data. (Have younger students graph one of the rounds; more advanced students can graph all rounds or the average of the rounds.)
- Conclude with a class discussion comparing results and graphs. What happened to the temperature as additional rounds were completed? (Answer: The water temperature continued to increase.) How would this relate to solar water heaters? (Answer: As the water moves through the solar heater it continues to warm.)
Vocabulary/Definitions
absorb: To be taken into a material without transmission or reflection.
active solar: Solar energy generating systems that require electrical or mechanical components, such as fans, pumps and electrical controls. These systems can be used for heating water or heating/cooling buildings.
convection: The transfer of thermal energy in a fluid (gas or liquid) by the circulation of currents in the heated fluid causing warmer packets to rise while cooler packets sink.
energy: The ability to do work.
heat exchanger: A device, such as an automobile radiator, that transfers heat from one liquid to another without allowing them to mix.
heat-transfer fluid: A fluid circulated in a heat exchanger. This fluid gains energy from one region and transfers it to another region.
insulation: A material used to prevent the passage of heat, electricity or sound; a non-conducting material.
parabolic: A specific type of curved shape used in solar trough collectors. The shape focuses the sun at 30 to 100 times its normal intensity, achieving temperatures of more than 400 °C. Having the form of a parabola — a plane curve formed by the intersection of a right circular cone and a plane parallel to an element of the curve.
passive solar: Solar energy generating systems that do not require electrical or mechanical components. These systems directly heat water or buildings, or reduce solar heat gain of buildings (for example, with window awnings to keep buildings cool).
pump: A machine or device for raising, compressing or transferring fluids.
radiation: The transfer of energy in the form of rays, waves or particles. A campfire and the sun both radiate heat and light.
renewable energy: Energy that is made from sources that can be regenerated. Sources include solar, wind, geothermal, biomass, ocean and hydro (water).
tank: A large container for holding or storing liquids or gases. For example, a gas tank in your car or a water heater tank.
Assessment
Pre-Activity Assessment
Discussion Questions: Solicit, integrate and summarize student responses. Ask the students how we could use solar energy to heat up water.
Activity Embedded Assessment
Worksheet: Have students record measurements and follow along with the activity on their worksheet. After they have finished their worksheet, have them compare answers with their peers. Review the worksheets to gauge their mastery of the subject.
Graphing: Using the worksheets, have students create graphs of how the temperature of the water changed over time.
Post-Activity Assessment
Re-Engineering: Ask the students how they could improve their solar heater and have them sketch or test their re-engineering ideas.
Concluding Discussion Questions: Ask the students and discuss as a class:
- How could an engineer or homebuilder make a simple solar water heater? (Possible answer: Paint barrels black and place them in an insulated container with a glass front.)
- Why should water storage tanks be well insulated? (Answer: This prevents heat from being transferred to the surroundings, which would cause the water in the tank to cool.)
- What is the source of water for a solar water heater (or any water heater)? (Answer: A well or city plumbing lines, called mains.)
- Would you expect the water that enters a solar water heater to be cold or hot? (Answer: Cold)
Troubleshooting Tips
To maximize water heating, adjust the setup to keep the flow of water through the tubing as slow as possible.
Complete the three or four rounds in quick succession because the water only gains about 5-9 ºF (3-5 ºC) and if left standing for several minutes, it cools down quickly.
Activity Extensions
Repeat the activity, but this time have some students use ice-cold water, some use warm water, and others use hot water. What is the final temperature for each? Compare the amount that the temperature changed in the three cases. Is it best if the fluid that enters a solar collector is cold, warm or hot? (Answer: Cold. This maximizes the rate of heat transfer to the fluid.)
Have the students conduct the activity outside on a sunny day. Have them think about the angle of their solar water heaters in relation to the position of the sun. Discuss why engineers would need to know the position of the sun to maximize solar water collection.
Have students conduct research to determine the amount of water Americans use every day. How much hot water to do we use daily? For what purposes? Do people in other countries use as much water? As much hot water? What are some ways to conserve water and energy? Have students report on how much water they use at home and reference against home water use bills.
Activity Scaling
- For upper grades, after students use their data to graph water temperature as a function of time, have them use their graph to predict the water temperature at an intermediate time step.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn how the sun can be used for energy. They learn about passive solar heating, lighting and cooking, and active solar engineering technologies (such as photovoltaic arrays and concentrating mirrors) that generate electricity.

Students are introduced to passive solar design for buildings — an approach that uses the sun's energy and the surrounding climate to provide natural heating and cooling. They learn about some of the disadvantages of conventional heating and cooling and how engineers incorporate passive solar design...

Students learn about the nature of thermal energy, temperature and how materials store thermal energy. They discuss the difference between conduction, convection and radiation of thermal energy, and complete activities in which they investigate the difference between temperature, thermal energy and ...

With the help of simple, teacher-led demonstration activities, students learn the basic physics of heat transfer by means of conduction, convection and radiation. They also learn about examples of heating and cooling devices, from stove tops to car radiators, that they encounter in their homes, scho...
References
Dictionary.com. Lexico Publishing Group, LLC. Accessed December 20, 2005. (Source of some vocabulary definitions, with some adaptation) http://www.dictionary.com
Elementary School Lesson Plans and Fun Solar Facts. Infinite Power, Renewable Energy Lesson Plans, Texas State Energy Conservation Office. Accessed November 21, 2005. (Source of activity, Solar Water Heaters, for middle school) http://www.infinitepower.org/lessonplans.htm
Solar Water Heaters. Updated September 14, 2005. Your Home, A Consumer's Guide to Energy Efficiency and Renewable Energy, Energy Efficiency and Renewable Energy, U.S. Department of Energy. Accessed November 21, 2005. http://www.eere.energy.gov/consumer/your_home/electricity/index.cfm/mytopic=12850
Copyright
© 2005 by Regents of the University of Colorado.Contributors
Xochitl Zamora-Thompson; Sabre Duren; Jeff Lyng; Malinda Schaefer Zarske; Denise CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: October 1, 2021






User Comments & Tips