Quick Look
Grade Level: 8 (6-8)
Time Required: 30 minutes
Expendable Cost/Group: US $0.10 This activity also requires some non-expendable items; see the Materials List for details.
Group Size: 28
Activity Dependency:
Subject Areas: Physical Science, Science and Technology
NGSS Performance Expectations:

| MS-PS3-1 |
| MS-PS3-5 |
Summary
Demonstrations explain the concepts of energy forms (sound, chemical, radiant [light], electrical, atomic [nuclear], mechanical, thermal [heat], and states (potential, kinetic).
Engineering Connection
Energy exists in many forms all around us. Engineers find ways to capture and release that energy in forms that are most useful to create heat where required and the work done in many engineered devices. These devices allow us to live with all of the comforts we are used to, such as air conditioning, transportation vehicles, ect.
Learning Objectives
After this activity, students should be able to:
- Describe at least three examples of how energy is converted from one form to another.
- State the law of conservation of energy.
- Identify seven forms and two states of energy.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS3-1. Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Construct and interpret graphical displays of data to identify linear and nonlinear relationships. Alignment agreement: | Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object and grows with the square of its speed. Alignment agreement: | Proportional relationships (e.g. speed as the ratio of distance traveled to time taken) among different types of quantities provide information about the magnitude of properties and processes. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS3-5. Construct, use, and present arguments to support the claim that when the kinetic energy of an object changes, energy is transferred to or from the object. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Construct, use, and present oral and written arguments supported by empirical evidence and scientific reasoning to support or refute an explanation or a model for a phenomenon. Alignment agreement: Science knowledge is based upon logical and conceptual connections between evidence and explanations.Alignment agreement: | When the motion energy of an object changes, there is inevitably some other change in energy at the same time. Alignment agreement: | Energy may take different forms (e.g. energy in fields, thermal energy, energy of motion). Alignment agreement: |
Common Core State Standards - Math
-
Fluently add, subtract, multiply, and divide multi-digit decimals using the standard algorithm for each operation.
(Grade
6)
More Details
Do you agree with this alignment?
-
Use variables to represent quantities in a real-world or mathematical problem, and construct simple equations and inequalities to solve problems by reasoning about the quantities.
(Grade
7)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Energy is the capacity to do work.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Illustrate how systems thinking involves considering relationships between every part, as well as how the system interacts with the environment in which it is used.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
National Science Education Standards - Science
-
Energy is a property of many substances and is associated with heat, light, electricity, mechanical motion, sound, nuclei, and the nature of a chemical. Energy is transferred in many ways.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Electrical circuits provide a means of transferring electrical energy when heat, light, sound, and chemical changes are produced.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
In most chemical and nuclear reactions, energy is transferred into or out of a system. Heat, light, mechanical motion, or electricity might all be involved in such transfers.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
New York - Science
-
Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Construct, use, and present an argument to support the claim that when work is done on or by a system, the energy of the system changes as energy is transferred to or from the system.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Materials List
For teacher demonstrations (these items can vary, depending on availability of objects):
- balls (various weights, sizes)
- paper cups
- a few electrical or mechanical appliances
- battery-operated object
Introduction/Motivation
Energy exists in many forms all around us. The development of our modern society has been accomplished because scientists and engineers have learned to capture some of that energy and transform it into ways to do useful work. For example, the conversion of energy from a chunk of coal into steam and then into mechanical engines that can do heavy work was important in the 19th century and the industrial revolution.
Engineers must know where to "find" energy resources and then how to convert them into forms that are more useful for all of the machines and gadgets we use in our daily lives.
Look around this room: What tools or devices are using energy? Lights are a good example. They convert electric energy into light energy. What about this cup of water, (hold up a cup); does it have energy? It has a state of energy called potential energy because it is held up at an elevation. If the water is poured into a pail, the potential energy is released as the water moves with some velocity. This is a kinetic state of energy.
The goal of this class is to explore some critical terms that are needed for energy – forms of energy and states of energy.
Procedure
Before Class:
Organize all materials for the demonstrations; try out the demos to make sure they all work with your collected items.
With the students:
1. (Hold up a ball.) Does this ball have energy? (Expect students to say no.) (Then drop it.) Did it have energy as it fell? (Yes, work was done moving an object so energy must have been used.) Where did it come from? Can energy just be created and infused into the ball? (No. The ball DID have energy when it was held at a height above the floor. We call that potential energy. Since energy was put into lifting the ball into the air, aka calories were burned by the person lifting, we can see how the ball built up potential energy).
2. Introduce the concept of states of energy.
- potential (stored energy) (hold ball up)
- kinetic (energy in motion) (drop ball)
Consider giving students the equations for potential and kinetic energy to reinforce that mass, height and velocity affect the values.
PE = mass*gravity*height
KE = 1/2*mass*velocity2
3. Ask some exploratory questions with these demonstrations.
- If I drop a bowling ball and a golf ball from the same height, which has more potential energy? (the bowling ball) What about kinetic energy? (The bowling ball.)
- If I drop two golf balls from different heights, which has more PE? (The higher one.)
- If I drop one golf ball, and throw the other one down from the same height, which has more KE? (The thrown one because it has a higher velocity. Energy was put into getting the ball moving by the person.)
5. Reinforce the concept of potential and kinetic energy by doing a cup-crushing demo.
- Place a paper cup on the floor and hold a small weight or baseball, 6 inches above the cup.
- Drop the ball and point out that the ball starts out with potential energy and converts to kinetic energy,
- Repeat for a height of 12 inches and 36 inches. (Use some sort of tube or pipe to direct the weight so it stays on course!)
- Ask the students where the energy is coming from that is lifting the ball. Does it require more energy to lift the ball higher? (Answer: Calories burned by person lifting, and yes)
- Ask students to predict the behavior.
- Now use a bowling ball, or heavier weight.
- This is a good time to refresh (or introduce, if you did not get to it during the human power activity) the concept of acceleration due to gravity. Use the traditional "Newton experiment" with a baseball and a piece of crumpled paper.
- Have two students come to the front of the room; give one the ball and the other the paper (tightly crumpled). Ask the class to predict which one will fall to the floor faster when dropped? Why? (They both should hit the floor at the same time – the acceleration due to gravity is constant.)
6. All energy also has a FORM. The seven forms (NYS standards) are: sound, chemical, radiant (light), electrical, atomic (nuclear), mechanical, thermal (heat). Remembered as "SCREAM Today."
- sound – from vibration of sound waves
- chemical (fuel, gas, wood, battery)
- radiant (light) (note – this is part of the broader "electromagnetic" group)
- electrical energy (electrons move among atoms – as in the conductive wire of an electrical cord)
- atomic (nuclear) (from nucleus of atom)
- mechanical (walk, run)
- thermal (heat) (rub hands together)
Emphasize that electricity is a way or transporting energy, but is not an energy SOURCE.
7. Use various tools, appliances and materials to introduce students to the forms and states of energy. Possible demonstrations or discussion topics are electrical appliances (light bulb, blender, hairdryer, toaster, etc.); human movement; a fire; and a roller coaster. For at least a few of them, draw a process flow diagram that identifies the forms/states of energy going into the device and those coming out of the device. For example: 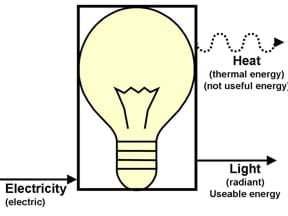
Point out:
- Some of the energy output from a device is the intended output – we want light from a light bulb. But we also sometimes get other forms of energy as output that is not desired. In this case, heat. When energy conversion devices are designed, engineers try to convert most of the energy that goes into the system into the intended output. Engineers are working to create light bulbs that produce more light energy and less heat. We describe these as more efficient light bulbs. That is one way to save energy – use more efficient devices. We'll learn more about efficiency in later lessons.
- Use a box to represent the item. Engineers do not usually draw the actual item when they are illustrating the flow of energy through an object.
Assessment
- If the mass of an object is 10 kg, and it is dropped from a height of 5 m, what is its potential energy? (Answer: PE=(10 kg)(9.8 m/s2)(5 m)=490 Nm) (A Nm (newton-meter) is equivalent to a (kg*m2)/s2)
- If the kinetic energy of an object is 100 Nm, and its velocity is 10 m/s, what is the mass of the object? (Answer: m=2KE/v2 =2*100Nm/〖10 m/s〗2 =2kg)
- Questioning: Use many opportunities throughout this activity to have students answer question related to forms or states of energy. The goal is to have students familiar enough with the terms and concepts so they can complete the energy conversion activity the following class period. That activity includes a student worksheet as a post-lesson assessment.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students participate in many demonstrations during the first day of this lesson to learn basic concepts related to the forms and states of energy. This knowledge is then applied the second day as students assess various everyday objects to determine what forms of energy are transformed to accomplish...

Students evaluate various everyday energy conversion devices and draw block flow diagrams to show the forms and states of energy into and out of the device.
Other Related Information
This activity was originally published by the Clarkson University K-12 Project Based Learning Partnership Program and may be accessed at http://internal.clarkson.edu/highschool/k12/project/energysystems.html.
Copyright
© 2013 by Regents of the University of Colorado; original © 2008 Clarkson UniversityContributors
Susan Powers, ; Jan DeWaters; and a number of Clarkson and St. Lawrence University students in the K-12 Project Based Learning Partnership ProgramSupporting Program
Office of Educational Partnerships, Clarkson University, Potsdam, NYAcknowledgements
This activity was developed under National Science Foundation grant nos. DUE 0428127 and DGE 0338216. However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: August 16, 2023




User Comments & Tips