Quick Look
Grade Level: 8 (6-10)
Time Required: 1 hours 15 minutes
(two 40-minute class periods)
Lesson Dependency:
Subject Areas: Physical Science, Science and Technology
NGSS Performance Expectations:

| MS-PS3-5 |

Summary
Students measure energy outputs and inputs to determine the efficiency of conversions and simple systems. One associated activity includes LEGO® motors and accomplishing work. The other investigates energy for heating water. Students learn about by-products of energy conversions and how to improve upon efficiency. Conduct either or both activities. The calculations in the water heating experiment are more complicated than in the LEGO motor activity, making the heating activity more suitable for older students, and only the LEGO motor activity suitable for younger students.Engineering Connection
Engineers always work to improve the energy efficiency of processes. This can sometimes be accomplished by recovering energy that is lost through inefficient energy conversions. For example, a typical cogeneration facility burns fuel to heat steam that turns electricity-generating turbines. The steam, partially cooled in the first step, is then used to heat homes and businesses. Cogeneration effectively combines two processes of electricity and heat production. Electric generation is about 30-40% efficient, while adding thermal production in a cogeneration facility can result in overall efficiencies of 80-90%.
Learning Objectives
After this lesson, students should be able to:
- Explain where energy is "lost" in conversions and why, based on the second law of thermodynamics.
- Compute the efficiency of an energy conversion given input and output.
- Identify system by-products and explain how they can be used effectively to increase overall system efficiency.
- Design a simple energy conversion system and test its efficiency.
- Use data they have collected to calculate the efficiency of a system.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS3-5. Construct, use, and present arguments to support the claim that when the kinetic energy of an object changes, energy is transferred to or from the object. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Construct, use, and present oral and written arguments supported by empirical evidence and scientific reasoning to support or refute an explanation or a model for a phenomenon. Alignment agreement: Science knowledge is based upon logical and conceptual connections between evidence and explanations.Alignment agreement: | When the motion energy of an object changes, there is inevitably some other change in energy at the same time. Alignment agreement: | Energy may take different forms (e.g. energy in fields, thermal energy, energy of motion). Alignment agreement: |
Common Core State Standards - Math
-
Find a percent of a quantity as a rate per 100 (e.g., 30% of a quantity means 30/100 times the quantity); solve problems involving finding the whole, given a part and the percent.
(Grade
6)
More Details
Do you agree with this alignment?
-
Fluently add, subtract, multiply, and divide multi-digit decimals using the standard algorithm for each operation.
(Grade
6)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Energy can be used to do work, using many processes.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Power is the rate at which energy is converted from one form to another or transferred from one place to another, or the rate at which work is done.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
National Council of Teachers of Mathematics - Math
-
solve problems that arise in mathematics and in other contexts
(Grades
Pre-K -
12)
More Details
Do you agree with this alignment?
-
recognize and apply mathematics in contexts outside of mathematics
(Grades
Pre-K -
12)
More Details
Do you agree with this alignment?
-
work flexibly with fractions, decimals, and percents to solve problems
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
understand and use ratios and proportions to represent quantitative relationships
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
select appropriate methods and tools for computing with fractions and decimals from among mental computation, estimation, calculators, or computers, and paper and pencil, depending on the situation, and apply the selected methods
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
develop, analyze, and explain methods for solving problems involving proportions, such as scaling and finding equivalent ratios
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
relate and compare different forms of representation for a relationship
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
model and solve contextual problems using various representations, such as graphs, tables, and equations
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
understand both metric and customary systems of measurement
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
use common benchmarks to select appropriate methods for estimating measurements
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
select and apply techniques and tools to accurately find length, area, volume, and angle measures to appropriate levels of precision
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
solve problems involving scale factors, using ratio and proportion
(Grades
6 -
8)
More Details
Do you agree with this alignment?
National Science Education Standards - Science
-
Use appropriate tools and techniques to gather, analyze, and interpret data. The use of tools and techniques, including mathematics, will be guided by the question asked and the investigations students design. The use of computers for the collection, summary, and display of evidence is part of this standard. Students should be able to access, gather, store, retrieve, and organize data, using hardware and software designed for these purposes.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Develop descriptions, explanations, predictions, and models using evidence. Students should base their explanation on what they observed, and as they develop cognitive skills, they should be able to differentiate explanation from description--providing causes for effects and establishing relationships based on evidence and logical argument. This standard requires a subject matter knowledge base so the students can effectively conduct investigations, because developing explanations establishes connections between the content of science and the contexts within which students develop new knowledge.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Think critically and logically to make the relationships between evidence and explanations. Thinking critically about evidence includes deciding what evidence should be used and accounting for anomalous data. Specifically, students should be able to review data from a simple experiment, summarize the data, and form a logical argument about the cause-and-effect relationships in the experiment. Students should begin to state some explanations in terms of the relationship between two or more variables.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Use mathematics in all aspects of scientific inquiry. Mathematics is essential to asking and answering questions about the natural world. Mathematics can be used to ask questions; to gather, organize, and present data; and to structure convincing explanations.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Mathematics is important in all aspects of scientific inquiry.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Energy is a property of many substances and is associated with heat, light, electricity, mechanical motion, sound, nuclei, and the nature of a chemical. Energy is transferred in many ways.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Heat moves in predictable ways, flowing from warmer objects to cooler ones, until both reach the same temperature.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Perfectly designed solutions do not exist. All technological solutions have trade-offs, such as safety, cost, efficiency, and appearance. Engineers often build in back-up systems to provide safety. Risk is part of living in a highly technological world. Reducing risk often results in new technology.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
-
Technological solutions have intended benefits and unintended consequences. Some consequences can be predicted, others cannot.
(Grades
5 -
8)
More Details
Do you agree with this alignment?
New York - Math
-
Fluently add, subtract, multiply, and divide multi-digit decimals using the standard algorithm for each operation.
(Grade
6)
More Details
Do you agree with this alignment?
-
Find a percent of a quantity as a rate per 100 (e.g., 30% of a quantity means 30/100 times the quantity); solve problems involving finding the whole, given a part and the percent.
(Grade
6)
More Details
Do you agree with this alignment?
New York - Science
-
Construct, use, and present an argument to support the claim that when work is done on or by a system, the energy of the system changes as energy is transferred to or from the system.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/cla_lesson6_efficiency] to print or download.Introduction/Motivation
Review combustion demo done in earlier conversions lesson.
- Does all of the heat go into heating the water and/or spinning the turbine? Where is the energy lost (at least lost from our ability to do work)? Where does this energy go?
- Reintroduce the first law of thermodynamics:
The law of conservation of energy: Energy can neither be created nor destroyed (by ordinary means); it can only be converted among the various forms.
So how do we "lose" energy? We don't – we just don't recover all of it in a usable form following energy conversion processes. Sometimes we want to capture the work (for example, moving a car), but also generate heat in the process that is not captured and used (in this case – the engine does not capture and use all of the heat energy released in the internal combustion engine).
Introduce efficiency:
- What does it mean when you are more efficient? (Listen to student suggestions.) Yes - you get the job done using less energy!
- What does "fuel efficiency "in automobiles mean? (More efficient cars use less gas to travel the same distance/speed.) Why do we care? (If possible show photographs of various cars, such as hybrids, or different systems that are more/less efficient.)
- Efficiency is a measure of how well our system works. That is, how much of the energy that is consumed is actually converted into a form that is useful to us.
- Efficiency = 100% * useful energy output / energy input (Stress the terms input and output!!) We must be able to measure energy in some fashion in order to calculate efficiency.
- Why is it important to consider the efficiency of our energy systems? (Open question for discussion; for example, it helps us save natural resources, requires less effort and energy if we have a more efficient system.)
Review efficiency calculation: Efficiency = (useful energy out/energy in) x 100%
Lesson Background and Concepts for Teachers
At this point in the unit, students have seen many of the components and concepts required for this lesson. They have drawn block flow diagrams with energy inputs and outputs identified and they should be familiar with the concept that energy can be "lost" in a form that is not useable for the intended work or heating task. The goal of this lesson is to take these concepts a step further in a quantitative approach. The specific concepts essential to this lesson include:
- The efficiency of a system is defined as the ratio of the useful output energy (or power) to the input energy (or power). These can be measured and calculated.
- The second law of thermodynamics can describe the energy that cannot be captured and used by humans.
- The efficiency of a system decreases as the number of energy conversions increases.
- A goal of technology is to increase efficiency, both directly and indirectly.

The term "thermodynamics" comes from two root words: "thermo," meaning heat, and "dynamic," meaning power. Thus, the laws of thermodynamics are the laws of "heat power."
First Law of Thermodynamics is commonly known as the Law of Conservation of Matter can also be applied to energy. It states that matter (energy) cannot be created and cannot be destroyed. The quantity of matter(energy) remains the same, but it can change form. The total amount of matter and energy in the universe remains constant.
The Second Law of Thermodynamics helps to explain energy (or mass) "losses." It is commonly known as the Law of Increased Entropy. While the first law tells us that the quantity of energy remains the same (First Law), the quality of energy deteriorates gradually over time as it gets dispersed in unusable forms. The usable energy is irretrievably lost from productive activities.
"Entropy" is a measure of how dispersed and unusable the energy becomes over time. As usable energy decreases, as in an inefficient energy conversion process, and unusable energy increases, "entropy" increases.
The first activity, Efficiency of an Electromechanical System, requires an understanding of the basic equations of force, work and energy (lesson 2) to simplify the measurements and calculations. In this activity, students provide energy into a Lego motor (on left in Figure 1), making it a generator. The electricity produced then powers a second motor (on right in Figure 1) that does work by raising a washer. The distance both washers rise can be easily measured.
In theory, both washers would rise to the same height if the system was 100% efficient. If we define the "useful energy output" as the work done to lift the right washer, then the basis for this experiment can be summarized as:
The energy into the system can be estimated from the work done to raise the left washer by twisting the large knob on the left.
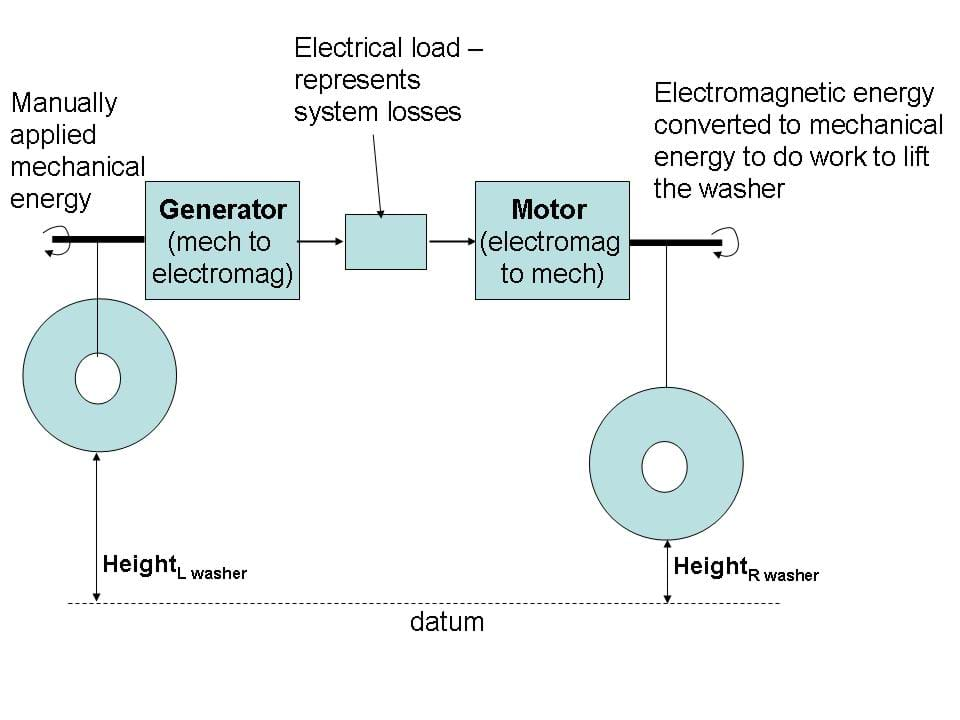
energy in = work to move left washer = force x distance = (masswasherg)heightLwashermoved
energy out = work to move right washer = force x distance = (masswasherg)heightRwashermoved moved
the efficiency defined by energy out/energy in:
efficiency(%) = 100% x(masswasherg)heightRwashermoved/(masswasherg)heightLwashermoved = 100% x heightRwashermoved/ heightLwashermoved
Refer to the Efficiency of a Water Heating System activity to have students conduct a hands-on experiment to solidify their understanding of efficiency.
These heights are illustrated in Figures 2 and 3.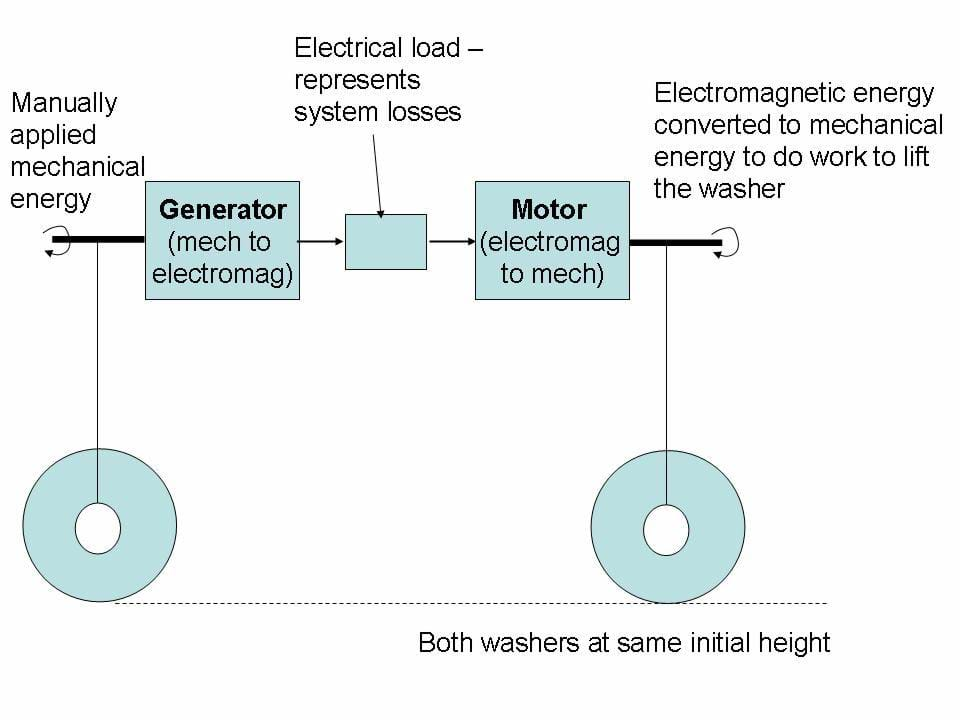
Associated Activities
- Efficiency of an Electromechanical System - Students use LEGO motors and generators to raise washers a measured height. The work done by the motor-generator systems is compared with the energy input.
- Efficiency of a Water Heating System - Students use a watt meter to measure energy input into a hot plate or hot pot used to heat water. The theoretical amount of energy required to raise the water by the measure temperature change is calculated and compared to the electrical energy input to calculate efficiency.
Vocabulary/Definitions
entropy: A thermodynamic measure of how dispersed and unusable energy becomes over time as it is converted between forms.
generator: A machine that converts mechanical energy into electricity.
motor: A machine that converts electricity into mechanical energy, generally for a rotational device.
second law of thermodynamics: Also known as the law of increased entropy, helps to explain energy (or mass) "losses." It explains why the quality of energy deteriorates gradually over time as it gets dispersed in unusable forms. The usable energy is irretrievably lost from productive activities.
thermodynamics: The study of energy; derived from the Greek roots "heat" and "power."
Assessment
Worksheets: Collect and review the student worksheets for both activities to make sure the calculations are done correctly and the answers to discussion questions show some insight.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students use a watt meter to measure energy input into a hot plate or hot pot used to heat water. The theoretical amount of energy required to raise the water by the measure temperature change is calculated and compared to the electrical energy input to calculate efficiency.

Students use LEGO® motors and generators to raise washers a measured height. They compare the work done by the motor-generator systems with the energy inputs to calculate efficiency.
References
All About Science – Second Law of Thermodynamics. Accessed December 30, 2008. http://www.allaboutscience.org/second-law-of-thermodynamics.htm
Other Related Information
This lesson was originally published by the Clarkson University K-12 Project Based Learning Partnership Program and may be accessed at http://internal.clarkson.edu/highschool/k12/project/energysystems.html.
Copyright
© 2013 by Regents of the University of Colorado; original © 2008 Clarkson UniversityContributors
Susan Powers; Jan DeWaters; Nate Barlow; and a number of Clarkson and St. Lawrence University students in the K-12 Project Based Learning Partnership ProgramSupporting Program
Office of Educational Partnerships, Clarkson University, Potsdam, NYAcknowledgements
This lesson was developed under National Science Foundation grant nos. DUE 0428127 and DGE 0338216. However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: August 16, 2023




User Comments & Tips