Summary
Students make two different formulations of imitation Silly Putty with varying degrees of cross-linking. They witness how changes in the degree of cross-linking influence the putty properties.
Engineering Connection
Materials engineers and more specifically, plastics engineers combine their knowledge of chemistry and material science to design, develop and manufacture new materials that have special properties to meet new applications. For example, to make aircraft, cars and electronic parts lighter and from recycled materials to reduce waste challenges engineers to make "smart polymers" that change their properties according to their environment. Such materials can be sensitive to many factors, such as temperature, humidity, pH, light intensity or electrical and magnetic fields, and can respond in various ways, such as altering color or transparency, becoming conductive or permeable to water or changing shape (shape memory polymers).
Learning Objectives
After this activity, students should be able to:
- Describe the role of cross-linkers in changing the physical properties of polymers.
- Design an experimental procedure for a design challenge.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed. Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate scientific and technical information (e.g. about the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. Alignment agreement: | Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of engineering design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Illustrate principles, elements, and factors of design.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Apply a broad range of making skills to their design process.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Matter has definite structure that determines characteristic physical and chemical properties
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 3 Dixie cups (small, wax-coated paper cups)
- pen or marker, to label cups
- 1 glass stirring rod
- 1 10 mL graduated cylinder
- 3 paper plates
- 1 meter stick
- Silly Putty Worksheet, one per student
To share with the entire class:
- 1 box borax, also known as saturated sodium tetraborate (Na2B4O7•10 H2O) (available in the laundry aisles of grocery stores)
- 1 liter 4% polyvinyl alcohol (available at chemical supply companies, such as VWR or Fisher Scientific)
- 1 gallon white glue, such as Elmer's Glue
- liquid food coloring
- 1 gallon distilled water
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/csu_polymer_lesson01_activity1] to print or download.Pre-Req Knowledge
A familiarity with polymeric materials, polymerization and cross-linking. This activity is designed to follow the Everyday Polymers lesson.
Introduction/Motivation
Who has played with Silly Putty? The recipe for Silly Putty was created by James Wright of General Electric in an attempt to make a synthetic rubber compound. During World War II, Japan occupied many of the natural rubber manufacturing countries in the Far East and cut off supply to the US. This began to hamper war production efforts, especially the manufacture of tires and boots. Although his invention of was not found to have good practical uses, Silly Putty did find success as a children's toy in 1949!
Today, we are going to follow in James Wright's footsteps and use poly (vinyl alcohol) and sodium tetraborate (borax) solutions to make a slimy polymer, resembling Silly Putty! Did you know that poly (vinyl alcohol), the main ingredient in Elmer's Glue, is a long chain of repeating ethyl alcohol monomers? You can think of these ethyl alcohol monomers -(CH2-CHOH)- as long strands of spaghetti. Sodium tetraborate is an ionic molecule that acts as a linker between the long chains of the polymers (draw Figure 1 on the board). Changing the ratios of polymer and sodium tetraborate solutions in your imitation Silly Putty will affect how it bounces, stretches and goos!
Procedure
Background
Students synthesize polymeric materials and create two different formulations of imitation Silly Putty. Then they design new versions of the putty that satisfies one of two different requirement options: very stretchy or rigid enough to bounce well. Students make their different formulations of poly(vinyl alcohol) by adding different amounts of sodium tetraborate (saturated borax solution). Figure 1 shows how the sodium tetraborate, the active ingredient in borax, facilitates the formation of tetrahydroxy borate through a boric acid intermediate (Note: A chemical intermediate is a reaction product of one or more steps in a step-wise reaction that is not a final product in the chemical reaction. Typically, chemical intermediates are quite reactive. These chemical intermediates help explain the step-wise mechanism for the formation of the borax cross-link detailed in Figure 2.)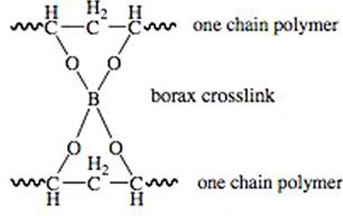
The step-wise mechanism in Figure 2 shows how sodium tetraborate in water produces boric acid. In the first step, sodium tetraborate dissociates into sodium cations and tetraborate anions. The tetraborate anion reacts with water to produce boric acid and two hydroxide anions. Then the boric acid reacts with one hydroxide ion to produce tetrahydroxy borate anion. The tetrahydroxy borate anion reacts with two alcohol groups in the poly(vinyl alcohol) backbone to make two covalent bonds linking the tetraborate ion with one polymer chain. The two remaining hydroxyl groups attach to the boron and then react with two additional alcohol groups within an adjacent polymer chain. This reaction completes the borax cross-link and creates a covalent bond that links these polymer chains together. As the amount of tetrahydroxy borate anion shown in the third step-wise reaction increases, a higher probability exists to form more borax cross-links within the poly(vinyl alcohol). When creating their putties, students can increase the amount of tetrahydroxy borate anion by adding more sodium tetraborate solution or by stirring more vigorously to disseminate the tetrahydroxy borate anion throughout the poly(vinyl alcohol) matrix. Either way, by forming more cross-links between poly(vinyl alcohol) chains, the material properties of the poly(vinyl alcohol) change, allowing students to witness their ability to influence the putty material properties.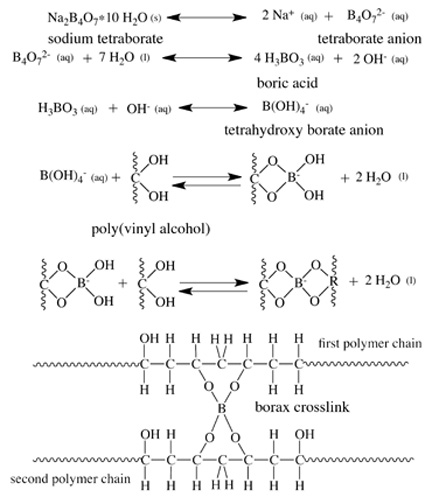
Before the Activity
- Prepare a saturated sodium tetraborate solution by dissolving small amounts of the borax powder (add 1 teaspoon at a time) into 100 mL distilled water, until only a small amount of borax powder remains at the bottom of the flask.
- Make copies of the Imitation Silly Putty Worksheet, one per student.
With the Students
- Divide the class into groups of four students each.
- Hand out materials to each group. Direct groups to go through the following steps.
- Direct groups through the following steps to make marked cups that they will use to measure the correct amounts of other ingredients: Use a graduated cylinder to add 10 mL of water to a Dixie cup. With a pen, mark the level of the water on the outside of the cup. Add an additional 10 mL of water to the cup and mark the water level. Repeat this process two more times, until a total of 40 mL has been added to the cup. Then pour out the water.
Formulation 1: 4% Poly(vinyl alcohol)
- Using the marked Dixie cup, measure 20 mL of 4% poly(vinyl alcohol) solution directly into the cup, followed by 3-4 drops of food coloring, if desired.
- Add 10 mL of the saturated sodium tetraborate solution to the colored poly(vinyl alcohol) solution and stir immediately! (Suggest that students have one group member continually stir while another adds the borate solution.)
- Remove the putty from the Dixie cup (leaving any excess water in the cup) and knead the putty on a paper plate until the desired consistency is reached. The paper plate helps to absorb excess water.
Formulation 2: Glue, 20% Poly(vinyl alcohol)
- Measure 10 mL of water into the Dixie cup and add 3-4 drops of food coloring.
- Using marked Dixie cup, add 10 mL of glue directly to the cup. Gently stir until the mixture has a uniform consistency.
- Add 10 mL of the saturated sodium tetraborate solution to the diluted glue solution and stir immediately!
- Remove the putty from the Dixie cup (leaving any excess water in the cup) and knead the putty on a paper plate until the desired consistency is reached.
Observations:
Write on the classroom board the table in Figure 3. Direct students to record their observations about the starting reagents and each polymer formulation produced. Record these observations in bullet or picture form. Be sure to include any observations about the starting reagents before mixing and then record all observations after the two reagents are mixed. Use these observations to help your team create a better formulation for the design challenge.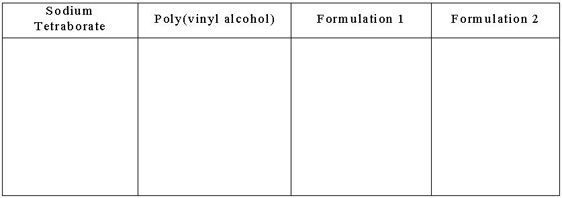
Design Challenge:
- Tell students that using what they have already learned, their engineering design challenge is to create a polymer that optimized for either 1) bounce or 2) stretch. Direct them to redesign or modify the procedure for making the putty to yield a material that is either elastic (stretchy) or rigid (bouncy). In order to do develop a solution to this challenge, students should experiment with different ratios of poly(vinyl alcohol) and saturated sodium tetraborate (borax) solution.
- Each groups' final product will be evaluated using measurements taken during three test trials. For the bounce test, putty will be dropped from 15 cm (do not throw the putty, this disqualifies a bounce), and the total centimeters of bounce from the ground is recorded. For the stretch test, you will hold one end of the putty and let gravity stretch the material, measuring the length (in centimeters) the putty reaches before breaking. Stretching the putty with hands is a disqualification.
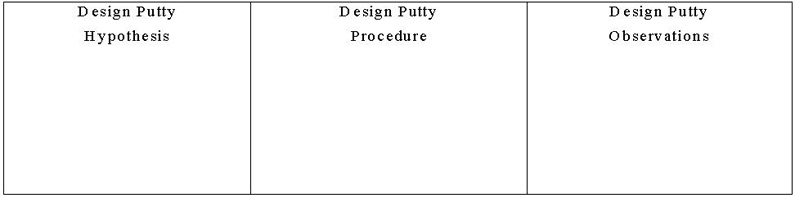
Figure 4. Example table for design putty hypothesis, procedure, and observations. - Direct students to create a table (see Figure 4) to document their hypotheses, procedures and observations/results.
Reflection:
- Did you redesign formulation 1 or 2? Explain how you redesigned the formulation procedure.
- Describe the material properties of the silly putty made using your redesigned procedure. Is the formulation more or less rigid or elastic?
- Are these material properties expected based on the changes you made to the original formulation procedure?
- How did you redesign formulation 1 or 2 perform in the bounce or stretch test? Please include the exact distance in centimeters.
- How would you redesign your formulation further to improve your material properties so your your silly putty would perform even better in the design challenge test.
(Note: Student responses will vary. In order to do develop a solution to this engineering design challenge, students should experiment with different ratios of poly(vinyl alcohol) and saturated sodium tetraborate (borax) solution. Depending on how they manipulate this ratio, the properties of their polymeric material change from either stretcher to bouncier.)
Conclusion:
- Bring together the class to share information on how groups approached the design challenge. Ask groups to briefly explain to the class: 1) how they decided to modify the procedure to design silly putty formulations that satisfied condition 1 or 2, including a demonstration of their putties by stretching or bouncing, 2) why their experimental modifications were successful or not in fulfilling the design challenge, and 3) what they would do to improve their putty formulations.
- Conclude by explaining to students that the design experimentation done by them in this activity is commonly done in industry when companies create new materials. Typically, the first formulation is not the best for the given application. Successful product creation requires numerous iterations (re-designs). For example, many different formulations of synthetic rubber were pursued before a viable one was discovered and optimized to replace the natural rubbers used in many consumer products.
Vocabulary/Definitions
cross-link: A covalent bond linking two polymeric chains together, sometimes facilitated by another molecule.
monomer: Building blocks that can be combined in various patterns to form different types of polymers.
polymer: A material made of monomer units.
Assessment
Pre-Activity Assessment
In-Class Questions: Quiz students on the material presented to them in the associated lesson. Listen to their answers to gain a sense of their familiarity with the topic.
Activity Embedded Assessment
Worksheet: During the activity, have students fill in their worksheets. Review their answers to gauge their comprehension.
Post-Activity Assessment
Design Challenge Reflection: Have students answer the following questions. Review their answers to gauge their comprehension of the material.
- How does the addition of sodium borate change the physical properties of the poly(vinyl alcohol) solution?
- Draw a schematic of a polymer that would be stretchy or one that would bounce well.
- Where can you find polymeric materials?
- Give an example of a commercial product that is made from a polymer.
- In your own words, describe a polymer.
- What happens when a cross-linker is added to a polymer material?
- What are the physical properties you observed about saturated sodium tetraborate solution?
- Prior to the experiment, what was your hypothesis for how the sodium tetraborate would affect the poly (vinyl alcohol) solution?
- Do you think the cross-linking event is a physical or chemical reaction? Explain your reasoning.
- In your own words, describe how sodium tetraborate interacts with the poly(vinyl alcohol) chains in formulation 1 and formulation 2?
Investigating Questions
- How does the addition of sodium borate change the physical properties of the poly(vinyl alcohol) solution?
- Draw a picture of a polymer that would be stretchy or one that would bounce well?
Safety Issues
- Sodium tetraborate (borax) is a bleaching agent and can burn eyes, thus, safety goggles must be worn at all times
- Do not taste, eat or lick the putty.
- Have students wash their hands after working with the putty.
Activity Extensions
At the point when students have completed the two primary polymer formations, pause and discuss as a class how these two formulations differ and in what ways are they similar. Brainstorm several different ways to modify the experimental procedure to vary the formulation to change the properties of the material.
Have students think about the concentration dependence of the desired material properties using formulation 1 and 2. The percent concentration of poly(vinyl alcohol) in formulation 1 to formulation 2 is very different. What material properties do students think will result from using a larger percent poly(vinyl alcohol)? Does it yield putty that better fulfills the design criteria or is the degree of cross-linking more important to the final properties of the putty?
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students explore the chemical identities of polymeric materials frequently used in their everyday lives. They learn how chemical composition affects the physical properties of the materials that they encounter and use frequently, as well as how cross-linking affects the properties of polymeric mater...
References
Borax. Part of Rio Tinto. Accessed August 8, 2011. http://www.borax.com
Ahluwalia, V. K. and Mishra, A. Polymer Science: A Textbook. Boca Raton, FL: CRC Press, 2008.
Alfrey, Tony. Slime. Science Experiments, Lucille M. Nixon Elementary School. Accessed August 4, 2011. (how to make slime) http://www.sci-experiments.com/slime/slime.html
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 Colorado State UniversityContributors
Cherelle M. Bishop; Kate McDonnell; Jeramy Jasmann; Melissa M. Reynolds; Michael A. de MirandaSupporting Program
CHIP GK-12 Project, Department of Electrical and Computer Engineering, Colorado State UniversityAcknowledgements
This work was developed by the Colorado Higher-Education Interdisciplinary Project (CHIP) in the Department of Electrical and Computer Engineering, based upon collaborative work supported by National Science Foundation grant no. 0841259. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Last modified: October 11, 2019




User Comments & Tips