Quick Look
Grade Level: 10 (10-12)
Time Required: 30 minutes
Lesson Dependency: None
Subject Areas: Chemistry, Physical Science
NGSS Performance Expectations:

| HS-PS2-6 |

Summary
Students explore the chemical identities of polymeric materials frequently used in their everyday lives. They learn how chemical composition affects the physical properties of the materials that they encounter and use frequently, as well as how cross-linking affects the properties of polymeric materials.Engineering Connection
Materials engineers, especially those who design plastics, combine an understanding of chemistry and material science to design, develop and manufacture new materials with special properties for new applications. For example, the demand for lightweight parts and vehicles using recycled materials challenges engineers to make "smart polymers" that change their properties according to their environments. Such materials can be sensitive to temperature, humidity, pH, light intensity, or electrical or magnetic field, and can respond in various ways, such as altering color or transparency, becoming conductive or permeable to water, or changing shape (shape memory polymers).
Learning Objectives
After this lesson, students should be able to:
- Describe how polymers are synthesized by combining two types of monomers with heat for a sufficient period of time.
- Predict how a cross-linker will cause a change in the physical properties of a polymer system and how molecular arrangements influence these materials properties.
- Describe examples how polymers are used in everyday life.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate scientific and technical information (e.g. about the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.Alignment agreement: | Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. Alignment agreement: | Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem. Alignment agreement: |
Common Core State Standards - Math
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Summarize, represent, and interpret data on a single count or measurement variable.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Colorado - Science
-
Matter has definite structure that determines characteristic physical and chemical properties
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/csu_polymer_lesson01] to print or download.Pre-Req Knowledge
A familiarity with basic chemistry concepts.
Introduction/Motivation
(Hold up a pair of sunglasses, a plastic bag, and a CD case for the class to see. Using Figure 2, write on the classroom board the chemical name and structure for each material. Then, talk about each item, highlighting its chemical structure. What atoms are in the structure? Do chemical groups exist in the backbone?)
Polymers are encountered in everyday life and are used for many purposes! Polymers are chains made of monomer subunits. A monomer is a repeating chemical unit. The structure and chemical composition of the polymer chain determines the physical properties of the material.
What are some items made from polymeric materials that you frequently use? (Listen to student responses.) Polymers are used to make electronic components, paint, plastic bottles, sunglass lenses, DVDs and so much more! Polymeric materials are usually derived from petroleum or oil, but significant research is underway to develop novel methods of producing these materials using renewable energy sources.
Materials engineers rely on some polymers for their rigid strength, others for their flexibility, and still others for their resistance to corrosion. For instance, poly(vinyl chloride) is a strong, corrosion-resistant polymer commonly used in plumbing applications, whereas polyethylene is an example of a flexible polymer found in plastic bags.
Lesson Background and Concepts for Teachers
(Present the following information to students as you show them the eight-slide Polymer Presentation, a PowerPoint file.)
Slide 1
- A polymer is a chemical term for a material composed of repeating units called monomers.
- Many consumer products are made from polymeric material. The polymeric material is formed by thousands of repeating monomers put together to make up a functional material.
Slide 2
- Some consumer products are made polymers, commonly called plastics. Just a few examples of the many, many polymeric materials are shown here.
- Not everything you see here has a polymeric composition. Can you guess what does? (Answer: The transparent portion of the Nalgene is polycarbonate, but the lids are not made from polycarbonate. The clear blue gel plastic on the running shoe is a polyurethane material designed to cushion a runners foot.)
Slide 3
- This animation is a simplistic representation of polymer synthesis, where monomers A and B are combined in a reaction vessel and then heated to create a polymer.
- At the end of the animation, a final polymer strand is show in an A-B-A-B pattern.
Slide 4
- Examples of a few commonly encountered consumer products made from polymeric materials.
Slide 5
- Some medical devices are made from polymers. For example, needles used for vaccines and IVs use a plastic casing typically made of polyurethane.
- (Bottom of slide from left to right:) The first two images show vascular grafts made from polytetrafluroethylene, with the second vascular graft (middle ) featuring roughness on the surface to promote integration of the vascular graft to the patient. The image on the right shows poly(vinyl chloride), commonly used in medical tubing.
Slide 6
- Introduce students to the different chemical players involved in making silly putty: monomer, cross-linker and polymer.
- A monomer is a basic building block of a polymer. Many vinyl alcohol monomers chemically linked yield a polymer called poly(vinyl alcohol).
- Poly(vinyl alcohol) is the polymer that students will use to make silly putty.
- In order to influence the material properties of the silly putty students make, they will need to determine the amount of tetrahydroxyborate anion or cross-linking agent that is incorporated into the polymer. A cross-linking agent is able to link poly(vinyl alcohol) chains together by forming a chemical bond.
Slide 7
- An illustration of the chemical link between two polymer chains.
- Note to teachrs: Refer to Figure 2 in the Imitation Silly Putty Worksheet. to understand how this chemical link is formed. You do not need to explain the figure to the students.
Slide 8
- This slide is very important to help students start thinking about the upcoming design challenge (in the associated activity).
- The "no cross-link" material has free-flowing polymer chains and stretches if force is applied to either end of the material chains.
- The opposite is true with the "with cross-link" material, because chemical linkers between changes prevent the individual polymer chains from stretching when a force is applied to either end, making a more ideal material to bounce.
- This information gives students an idea for how to approach the design challenge after they make two different formulations of a poly(vinyl alcohol) silly putty.
Synthetic and Natural Polymers
Polymers are large molecules held together by chemically linked subunits called monomers. The first scientist to discover that polymers contained many small repeating units (monomers) was Hermann Studadinger. Studadinger received the Nobel Prize in chemistry for his discovery of the chemical structure of natural rubber. Following the discovery of synthetic materials, scientists learned how to modify and tune their chemical and physical properties to make them useful for various applications. 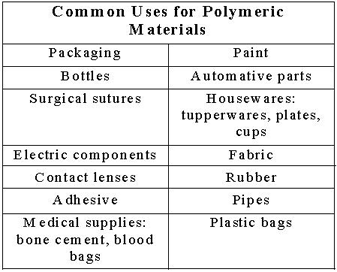
Figures 1 and 2 list just a few examples of the many ways that polymeric materials have changed our day-to-day lives through primarily low-cost, mass-produced materials. Many of these materials are made from synthetic polymers and were developed in commercial laboratories, but countless other polymer examples are found in nature and in living organisms. For example, Chitin, also known as N-acetylglucoseamine (derived from glucose monomers), forms the hard exterior of many crustaceans, turtle and beetle shells. Cellulose, a polysaccharide, is used by many plants for structural stability. Polymers also exist inside the human body. Proteins and DNA are both synthesized from small subunits, called amino acids.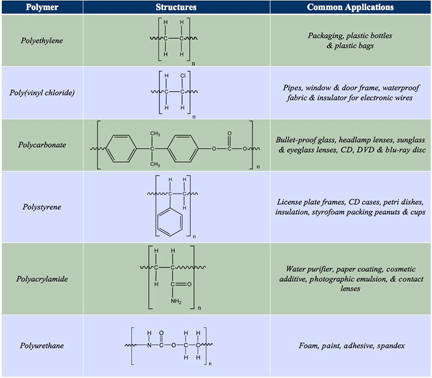
Polymerization
Polymers are formed by a process called polymerization, in which a chemical reaction of reacting monomer molecules forms polymer chains in a variety of complexities. Depending on the polymerization reaction conditions, the resulting polymer can be a simple linear chain of linked carbon atoms. Chain-growth polymerization (or addition polymerization) involves the linking together of molecules incorporating double or triple carbon-carbon bonds. The physical properties of the materials are influenced by the arrangement of the chains. A polymer network consists of many polymer chains connected through a number of covalent linkages called cross-links. Most of the polymers we talk about here are linear polymers. A linear polymer is composed of one molecule after another, hooked together in a long chain called the backbone. Now, linear polymers do not have to be in a straight, rigid line. Those single bonds between atoms in the backbone can swivel around a bit, like paper clips hooked together end-to-end. 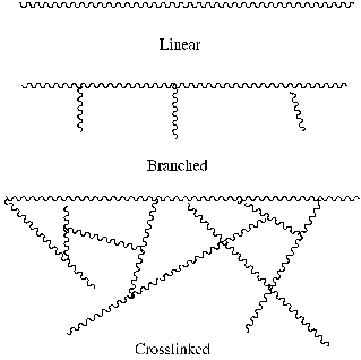
Molecular Forces and Chemical Composition of Polymers
Differences in chemical composition can influence a material's application. For example, the polymer polyethylene has an ethylene backbone (CH2CH2). Polyethylene is one of the most common polymeric materials found in plastic packaging, bottles and shopping bags. The uses of polyethylene are very different from another polymer, poly(vinyl chloride), which is commonly used for water pipes and is able to withstand large amounts of pressure. Other applications for poly(vinyl chloride) are door frames, waterproof fabric and electrial wire insulation, The only chemical difference between polyethylene and poly(vinyl chloride) is the substitution of one hydrogen atom in polyethylene for a chloride ion in poly(vinyl chloride), as shown in Figure 4. The inclusion of a chlorine atom introduces a change in the physical properties of the overall material. The next monomer unit, featured in Figure 2, is polycarbonate. 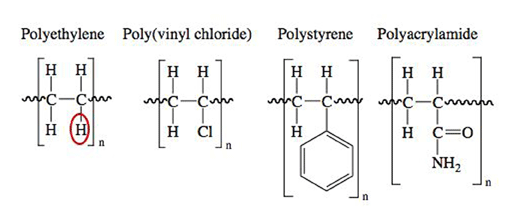
Polycarbonate is commonly used in bulletproof glass and headlights, in lenses for headlamps, glasses and sunglasses, as well as in CD, DVD and Blu-Ray discs. Polycarbonate monomer includes the integration of two, six-membered aromatic rings separated by a carbon atom that has two methyl groups attached. Aromatic rings (also known as aromatic compounds or arenes) are hydrocarbons that contain benzene. Benzene, C6H6, is often drawn as a ring of six carbon atoms, with alternating double bonds and single bonds as shown below.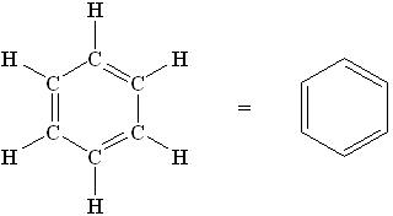
The inclusion of the aromatic rings can lead to pi-stacking between different polymer chains. In chemistry, pi stacking (also called π-π stacking) refers to attractive, non-covalent interactions between aromatic rings. This means that non-covalent interactions between pi-bond electrons available in one polymer chain can interact with aromatic rings on another polymer chain when they are in close proximity. Polycarbonate also includes an ester linkage within the polymer back-bone. Ester functional groups are a less polar functional group than an alcohol group. Polystyrene is found in many products including: license plate frames, CD cases, Petri dishes, insulation and Styrofoam packing peanuts. Polystyrene's monomer unit is similar to polyethylene and poly(vinyl chloride), in which a hydrogen atom in polyethylene monomer is substituted with a six-membered aromatic ring, in the case of polystyrene (see Figure 4). Ring stacking can also be observed between chains of polystyrene as with polycarbonate. Polyacrylamides are used in a various applications ranging from water purifiers, paper coating, cosmetic additives, photographic emulsion and contact lenses. Again, polyacrylamide can be derived from polyethylene by substituting a hydrogen atom with an amide functional group. The final monomer featured in Figure 2 is the monomer unit for polyurethane. Polyurethanes are used in foams, paints, adhesives and spandex. This monomer is similar to polycarbonate in that it cannot be derived from polyethylene's monomer unit. Polyurethanes are connected by urethane linkages.
Effect of Cross-Links
A cross-link is a covalent bond formed between two polymer chains in a material (see Figure 5). These covalent bonds cause the polymer chains within a polymeric material to become networked. A polymer network is a network in which all polymer chains are interconnected to form a single macroscopic entity by many crosslinks. In general, cross-links tend to make the polymer chain closer together and cause the material to become more rigid. In the associated activity, Let's Make Silly Putty, studentsuse hydroxy tetraborate to form four covalent bonds between two poly(vinyl alcohol) chains when making imitation Silly Putty. Depending on the degree of cross-linking within a material, the polymer chain will have different properties. When no cross-links are present to chemically link the chain together, the chains are able to move much more freely. Long-chain polymers often have many kinks in the chains, and these kinks can move and un-kink, causing the material to stretch. To illustrate this point, imagine a kink in a garden hose; the kinks loosen after enough force is applied, which is similar to how polymeric materials stretch. The act of stretching a polymer forces the polymer chain to align with each other because of the force applied to the material.
When many long polymer chains with a large number of cross-links are present within a material, the chains are chemically linked, making the material more rigid. Thus, the degree of cross-linking throughout a material is very important in understanding how the physical properties of the material change. In other words, more cross-linking within a polymeric material results in a more rigid material, whereas less cross-linking results in a more elastic material. 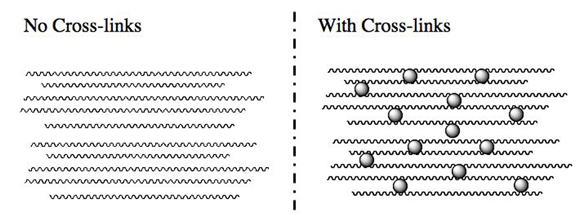
Associated Activities
- Let's Make Silly Putty - Students make two different formulations of imitation Silly Putty with varying degrees of cross-linking. They witness how changes in the degree of cross-linking influence the putty properties.
Lesson Closure
Remember, cross-linking affects polymeric material properties by limiting the motion of individual polymer chains within a material (as highlighted visually in Figure 5). We can see the effect of cross-linking on polymeric materials by comparing the strength of polyethylene and cross-linked polyethylene. Polyethylene, as discussed previously, is used to make plastic shopping bags and many plastic containers, whereas cross-linked polyethylene (abbreviated as PEX) tubing is used in plumbing applications. The strengths of a polyethylene plastic bag and a water pipe made from cross-linked polyethylene are very different. Cross-linking of the polymer chains strengthens the material properties present in PEX tuning.
Vocabulary/Definitions
copolymer: A polymer made from two or more types of monomer subunits.
cross-linker: A covalent bond linking two polymeric chains together, sometimes facilitated by a small molecule.
homopolymer: A polymer made from only one type of monomer.
monomer: The building block of polymers. Monomers can be combined in various repeating patterns to form different types of polymers.
natural polymer: A polymer that is synthesized naturally by a plant or organism.
polymer: A material composed of repeating monomer units.
polymerization: The process of chemically linking monomers in various patterns to produce a polymeric material.
synthetic polymer: A polymer was made by humans.
Assessment
Worksheet: Have students complete the Polymer Worksheet. Review their answers to gauge their comprehension.
Lesson Extension Activities
Recycling Drive: Ask students to each bring in several examples of polymeric materials for a class recycling drive. Use this opportunity to identify the seven recycling codes (see Figure 6) and discuss the chemical properties of each plastic material. Consumer products also typically use an initial stamp to signify the chemical compositions of the polymer used to create the material. Although recycling codes are usually placed on polymeric materials, not all polymeric materials used in consumer products are labeled with recycling coded and not all polymeric materials are actually recycled. Frequently, this is because some polymeric materials are extremely durable, making recycling of the material costly and time consuming. Another roadblock to recycling some plastics is non-existent demand for the post-recycled materials. To make the use of recycled materials more common in consumer products, it helps to increase the demand by purchasing recycled materials or products that use post-recycled plastic. Polymeric materials, as demonstrated in this lesson and in Figures 2 and 6, are not all chemically the same. This fact alone makes recycling all of these different plastic materials challenging, time consuming and costly. Another consideration is that many consumer products are made with blended polymeric materials, meaning that more than one polymer is used. These blended and composite plastics may not be able to use the same recycling process, making recycling costly and challenging for them. Some success has been achieved in recycling certain polymeric materials, such as polyethylene terephthalate (PETE), which is used to make plastic bottles, packaging and plastic containers. Its post-recycled material has found a niche application as a polyester insulation fiber used in winter jackets, pillows, bedding and carpeting. 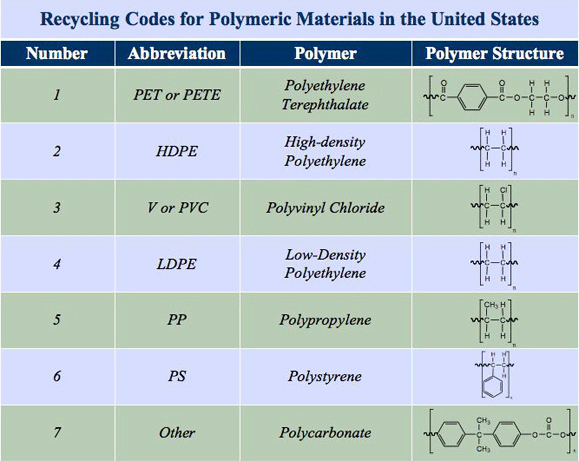
Research Projects: Have students (either in small groups or individually) research an assigned polymeric material, investigating its chemical properties, processing techniques and applications. Have students describe how the material's properties and processing techniques enable the polymer to function well in various applications. Optionally, ask students to include schematics of the polymer chains and describe how certain functional groups interact with other functional groups between different polymer chains. Use this as an opportunity to incorporate scientific writing guides, electronic researching tools, and scientific citation into the class.
Homework: Lead a class discussion about possible types of plastics that students use at home. Ask them to bring in examples not mentioned in the lesson presentation and talk about their polymer types and properties. Select polymeric materials based on the chemical structures mentioned in the PowerPoint presentation and summarized in Figure 2. Additionally, have students each locate a material made from a polymer and be prepared to explain its chemical structure to the class.
Additional Multimedia Support
Note: The animation in the attached PowerPoint presentation (v.11.5.5 or higher) requires Quicktime (v. 7.7 or higher).
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics.

Students make two different formulations of imitation Silly Putty with varying degrees of cross-linking. They witness how changes in the degree of cross-linking influence the putty properties.

Students are introduced to the circulatory system with an emphasis on the blood clotting process, including coagulation and the formation and degradation of polymers through their underlying atomic properties. They learn about the medical emergency of strokes—the loss of brain function commonly due ...

The Great Pacific Garbage Patch (GPGP) is an intriguing and publicized environmental problem. Through exploring this complex issue, students gain insight into aspects of chemistry, oceanography, fluids, environmental science, life science and even international policy.
References
Ahluwalia, V. K. and Mishra, A. Polymer Science: A Textbook. Boca Raton, FL: CRC Press, 2008.
Bahadur, P. and Sastry, N. V. Principles of Polymer Science. Boca Raton, FL: CRC Press, 2002; p. 401.
Morgan, N. "Polymers and Plastics." in Chemistry in Action: The Molecules of Everyday Life. New York, NY: Oxford University Press, 1995, pp. 94-107.
Nicholson, J. W. The Chemistry of Polymers. Third Edition. London, UK: Royal Society of Chemistry, 2006.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 Colorado State UniversityContributors
Cherelle M. Bishop; Kate McDonnell; Jeramy Jasmann; Melissa M. Reynolds; Michael A. de MirandaSupporting Program
CHIP GK-12 Project, Department of Electrical and Computer Engineering, Colorado State UniversityAcknowledgements
This work was developed by the Colorado Higher-Education Interdisciplinary Project (CHIP) in the Department of Electrical and Computer Engineering, based upon collaborative work supported by National Science Foundation grant no. 0841259. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Last modified: April 22, 2021





User Comments & Tips