Quick Look
Grade Level: 7 (6-8)
Time Required: 45 minutes
Expendable Cost/Group: US $1.00
Group Size: 2
Activity Dependency: None
Subject Areas: Biology, Chemistry, Life Science
Summary
Students use a simple pH indicator to measure how much CO2 is produced during respiration, at rest and after exercising. They begin by comparing some common household solutions in order to determine the color change of the indicator. They review the concepts of pH and respiration and extend their knowledge to measuring the effectiveness of bioremediation in the environment.
Engineering Connection
When toxic materials are spilled into the environment, engineers can use microorganisms, fungi or plants to clean up the spill through a process called bioremediation. The engineers choose an organism that can "eat" the target contamination. One way that engineers can tell if the bioremediation is working is by measuring how much the bacteria are "breathing." Engineers measure how much organisms are breathing by changes in pH of the soil or water in which they are growing. Measuring the results of cell activity is usually easier than trying to keep track of the actual amount of toxic material in the environment.
Learning Objectives
After this activity, students should be able to:
- Describe the effects of cellular respiration on pH.
- Explain how engineers use pH to measure cellular respiration in bioremediation of contaminated soils.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Analyze and interpret data to determine similarities and differences in findings.
(Grades 6 - 8)
More Details
Do you agree with this alignment?
-
Graphs, charts, and images can be used to identify patterns in data.
(Grades 6 - 8)
More Details
Do you agree with this alignment?
-
Cellular respiration in plants and animals involve chemical reactions with oxygen that release stored energy. In these processes, complex molecules containing carbon react with oxygen to produce carbon dioxide and other materials.
(Grades 6 - 8)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Display numerical data in plots on a number line, including dot plots, histograms, and box plots.
(Grade
6)
More Details
Do you agree with this alignment?
-
Summarize numerical data sets in relation to their context, such as by:
(Grade
6)
More Details
Do you agree with this alignment?
-
Reporting the number of observations.
(Grade
6)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Report the number of observations.
(Grade
6)
More Details
Do you agree with this alignment?
-
Summarize numerical data sets in relation to their context.
(Grade
6)
More Details
Do you agree with this alignment?
-
Display numerical data in plots on a number line, including dot plots, histograms, and box plots.
(Grade
6)
More Details
Do you agree with this alignment?
Colorado - Science
-
Gather, analyze, and interpret data regarding the basic functions of photosynthesis and cellular respiration
(Grade
7)
More Details
Do you agree with this alignment?
-
Use direct and indirect evidence to describe the relationship between photosynthesis and cellular respiration within plants – and between plants and animals
(Grade
7)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 4 small clear plastic cups
- 2 straws
- 1 spoon
- Breathing Bubbles Worksheet
To share with the entire class:
- water
- red cabbage indicator solution (Instructions: Chop a cabbage into small pieces and steep in boiling water for at least 10 minutes. Then, filter out the cabbage pieces using a coffee filter or tea strainer. You should be left with a bluish/purple solution at neutral pH.)
- 4 clear different solutions to measure pH (examples: diluted lemon juice and/or vinegar, baking soda mixed with water, water, soda pop, etc.)
- pH meter (if available) or pH paper strips (optional)
- plastic gloves (optional)
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_cells_lesson02_activity1] to print or download.Pre-Req Knowledge
Students should have a basic understanding of pH. It is also helpful to have a basic understanding of cellular respirations, as discussed in the associated lesson, Cellular Respiration and Bioremediation.
Introduction/Motivation
What do you know about pH? The pH of a solution is a measure of how much acid or base is in a solution. A low pH corresponds to an acidic solution, and a high pH corresponds to a basic solution. As a point of reference, a neutral pH would be 7. What is an example of a solution with a low pH? This would be anything acidic, such as citrus fruit or vinegar (remember: low pH equals high acidity). What solution has a neutral pH? Distilled water is a solution that has a perfectly neutral pH. What is an example of a solution that has a high pH, or is basic? Basic solutions would include baking soda, ammonia and bleach (remember: high pH equals low acidity, or is basic). pH measures the amount of hydrogen ions in a solution. Lots of hydrogen ions form an acidic solution, and fewer hydrogen ions form a basic solution.
Did you know that cellular respiration has a pH value? When a cell goes through cellular respiration, it consumes oxygen and produces CO2 which lowers the pH of water (forming an acidic solution). On the other hand, when cells go through photosynthesis, they produce oxygen, which raises the pH of water (forming a basic solution).
Bioremediation is a process whereby engineers use something living, like a microorganism, fungus or green plant, to return a polluted environment back to its original state. During bioremediation, some cells can use certain types of pollution as food for cellular respiration, to create energy for growth, life and reproduction. How do you think engineers can use pH to measure bioremediation? Well, pH tells us about the chemistry of water and soil. Engineers can test the pH of an area to determine if bacteria or other cells are growing and performing cellular respiration in the area. If the pH is very acidic, then cellular respiration may be occurring (or the water may be acidic due to the presence of inorganic acids). The organisms that engineers use for bioremediation are microscopic. So, it is hard to detect them directly. It is much less expensive and faster for engineers to measure the pH that bacteria cells produce when they grow and reproduce in the environment than to develop complex equipment to detect their presence. If you measure the pH of a polluted system and then add microorganisms in order to eat up the pollution, the pH of the system should decrease over time as the microbes do their job. This decrease in pH provides evidence that the bioremediation is proceeding as it should.
Today we are going to measure the pH of a variety of solutions and then measure how much CO2 we breathe out when we are resting and when we are exercising. First, we are going to test our pH indicator on four different solutions by adding a few drops of the indicator to each solution. Once we have determined which solutions are acidic, basic or neutral, we will try to identify the solutions as a class. Next, we will measure how much CO2 we produce when we are resting and exercising, using the same indicator we used to determine the identity of the four solutions. Lastly, we will think about how we can use pH to help engineers optimize bioremediation.
Procedure
Background
In this activity, students measure how many breaths it takes to change the color of the indicator to acidic from neutral when they are resting and then after they have been exercising. Their bodies naturally produce more CO2 when they have been exercising than when they are resting. The indicator should turn to acidic faster after they have been exercising.
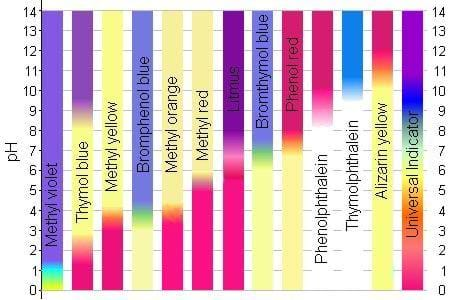
Cabbage indicator colors (see Figure 1):
- Acidic pH turns the cabbage juice red.
- Neutral pH keeps the cabbage juice purple.
- Basic pH turns the cabbage juice blue.
Before the Activity
- Gather materials and make copies of the Breathing Bubbles Worksheet.
- Make the red cabbage indicator solution the day before. (See instructions in Materials List section)
With the Students
- Pass out materials to students. Have them line up their four cups to receive the "unknown" solutions.
- Pour samples of four unlabeled solutions, one in each cup, to each team of students. Have students use the indicator to assign identities to four clear solutions by adding a spoonful of indicator into the solution until it turns color. For acidic solutions, the indicator should turn red, and for basic solutions the indicator should turn blue. Have students record their observations on their worksheets.
- Next, have students thoroughly rinse their cups. Pour cabbage juice indicator into all four of the cups, about halfway full.
- Remind students that CO2 is produced during cellular respiration and O2 is consumed. This results in an acidic solution being created. Have students record on their worksheets the color of the cabbage juice indicator before the experiment begins.
- Next have each student breathe into one cup of the indicator solution through the straw, remembering not to drink the indicator but blow into it. Record the number of breaths that it takes to turn the indicator to an acidic pH.
- Next, have students sprint, briskly walk up and down stairs, or do some jumping jacks.
- Have the students repeat the process of breathing into the indicator solution through the straw, and record how many breaths it takes to turn the solution to an acidic color after exercising.
- Have students report their results to the class and record the results on the board.
- Next, have students answer the results and engineering questions on their worksheets.
- As a class, discuss how engineers can use a similar technique to measure the amount of microbial activity in the water or in the soil where a toxic spill has occurred. Engineers can save time and money by measuring changes in pH after they have added bacterial cells or plants to a bioremediation site instead of trying to culture organisms in the lab.
Vocabulary/Definitions
carbon dioxide: (CO2) A gas at room temperature that is produced during cellular respiration; when bubbled into water, CO2 lowers the pH of the water creating an acid called carbonic acid.
oxygen: A gas that is consumed during cellular respiration.
pH: A measure of how much acid or base is in a solution; a low pH corresponds to an acidic solution, and a high pH corresponds to a basic solution. A neutral pH is equal to 7.
pH indicator: A solution that changes color depending on the surrounding pH.
Assessment
Pre-Activity Assessment
Class Discussion: Gauge the students' prior knowledge of the material by asking the following questions:
- What is pH? (Answer: The pH of a solution is a measure of how much acid or base is in the solution.)
- What is cellular respiration? (Answer: The process in which cells convert food into energy for growth, survival and reproduction.)
Activity Embedded Assessment
Worksheet/Pair Check: Have students record measurements and follow along with the activity on their worksheets. After students have finished their worksheets, have them compare answers with their peers.
Post-Activity Assessment
Engineering and Bioremediation Costs: Bioremediation has many cost and efficiency benefits. Have students think about how the expense and resources put into bioremediation might be different than developing tools and equipment to remove contaminants from soils and water. Have them write a paragraph or hold a class discussion comparing bioremediation vs. land removal in contamination cleanup. Use the following questions to frame the advantages and disadvantages of the two remediation options systematically:
- What materials are needed for land removal? (Excavation equipment, usually a backhoe to dig out the soil, a giant bin to store the excavated soil, and a large truck to transport the excavated soil to the appropriate disposal facility.)
- Which remediation option (bioremediation or land removal) do you expect to be less expensive? (There are fewer costs associated with bioremediation because biodegradation is a naturally occurring process. Engineers harness the ability of microbes or larger organisms such as plants to degrade contaminants during bioremediation.)
- Can we use bioremediation at any contaminated site? What conditions must be present for bioremediation to be effective? (Microbes must be present at the contaminated site in order for bioremediation to be possible. Alternatively, microbes can be introduced to the site, but the success rates with non-native microbes are much lower.)
- Which remediation option has a higher likelihood of failure? (There are more variables influencing the success of bioremediation; for example, if the soil conditions and the contaminant are not conducive to microbial growth, then bioremediation will not work. Land removal is more straightforward and predictable because to eliminate the contaminant, all you have to do is excavate the soil.)
To systematically evaluate the two remediation options, have students complete the chart below. The chart should help students to organize their thoughts from the previous discussion.
Engineering Recommendations: Have students pretend to be consultants for an engineering firm for one of the following scenarios. Ask the students to make recommendations about how to monitor the bioremediation of the area based on what they learned during this activity.
- A piece of land contaminated with heavy metals and oils from an old industrial factory.
- A former shipyard that has leaking barrels of oil in one area.
- A piece of farmland that has been previously treated with several harmful pesticides.
- A piece of land that has been contaminated with soaps and solvents used by a dry cleaning company.
- A nuclear waste site that has very high amounts of radioactive materials leeching into the soil and groundwater.
Safety Issues
Do not use any toxic liquids for your pH testing; students may inadvertently get some solution on their hands or in their mouths.
Although it is not harmful, remind students not to drink the indicator solution.
Troubleshooting Tips
The students need to thoroughly wash out their four cups after testing the unknown solutions. Any residue left in the cups may affect their breathing experiment.
If the indicator solution does not change color, the students can use pH paper to measure the pH of a solution of water after they breathe into it while resting and then after they have been exercising.
Activity Extensions
Have students learn more about monitoring the effects of bioremediation. Several measurements are usually performed, including oxidation reduction potential (redox), pH, temperature, and oxygen content.
Use the same pH indicator to measure the "breathing" of yeast cells. Have the class grow yeast cells in a solution of warm water and sugar and add indicator to the yeast solution in order to watch the pH change as the yeast cells produce acid when they digest the sugar.
Activity Scaling
- For upper grades, have students describe pH and indicators quantitatively. Have them create scales for measuring the pH of different solutions using the cabbage juice indicator.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to acids and bases, and the environmental problem of acid rain. Students also conduct a simple experiment to model and discuss the harmful effects of acid rain on our living and non-living environment.

Students take advantage of the natural ability of red cabbage juice to perform as a pH indicator to test the pH of seven common household liquids. Like environmental engineers working on water remediation or water treatment projects, understanding the chemical properties (including pH) of contaminan...

Students learn about the basics of cellular respiration. They also learn about the application of cellular respiration to engineering and bioremediation. And, they are introduced to the process of bioremediation and examples of how bioremediation is used during the cleanup of environmental contamina...
References
Helmenstine, Anne Marie. How to make red cabbage pH indicator: Acid base chemistry. Accessed August 23, 2008. http://chemistry.about.com/library/weekly/aa012803a.htm
Copyright
© 2008 by Regents of the University of Colorado.Contributors
Kaelin Cawley; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: April 29, 2024




User Comments & Tips