Quick Look
Grade Level: 9 (9-11)
Time Required: 4 hours
Four 60-minute periods over four days
Expendable Cost/Group: US $10.00
Group Size: 3
Activity Dependency: None
Subject Areas: Algebra, Physical Science, Physics, Problem Solving
NGSS Performance Expectations:

| HS-PS3-3 |

Summary
Student teams design and build solar water heating devices that mimic those used in residences to capture energy in the form of solar radiation and convert it to thermal energy. This thermal energy is next transferred to water (to be used as domestic hot water) in the form of heat. In doing this, students gain a better understanding of the three different types of heat transfer, each of which plays a role in the solar water heater design. Once the model devices are constructed, students perform efficiency calculations and compare designs.Engineering Connection
Many of today's engineers focus their efforts on how to incorporate alternative forms of energy into our homes and buildings as a replacement for burning fossil fuels, which have a harmful effect on our environment. One way is to take advantage of the radiation provided by our sun, converting it to thermal energy to generate electricity, heat water and cook food.
Learning Objectives
After this activity, students should be able to:
- Identify locations in the solar water heater at which each type of heat transfer is being utilized.
- Explain why solar energy is a good alternative to the combustion of natural gas.
- Explain how the engineering concepts in this design project can be applied to solve real-world problems.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS3-3. Design, build, and refine a device that works within given constraints to convert one form of energy into another form of energy. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design, evaluate, and/or refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | At the macroscopic scale, energy manifests itself in multiple ways, such as in motion, sound, light, and thermal energy. Alignment agreement: Although energy cannot be destroyed, it can be converted to less useful forms—for example, to thermal energy in the surrounding environment.Alignment agreement: Criteria and constraints also include satisfying any requirements set by society, such as taking issues of risk mitigation into account, and they should be quantified to the extent possible and stated in such a way that one can tell if a given design meets them.Alignment agreement: | Energy cannot be created or destroyed—it only moves between one place and another place, between objects and/or fields, or between systems. Alignment agreement: Modern civilization depends on major technological systems. Engineers continuously modify these technological systems by applying scientific knowledge and engineering design practices to increase benefits while decreasing costs and risks.Alignment agreement: |
Common Core State Standards - Math
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
A prototype is a working model used to test a design concept by making actual observations and necessary adjustments.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Evaluate ways that technology can impact individuals, society, and the environment.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Colorado - Science
-
Gather, analyze and interpret data on chemical and physical properties of elements such as density, melting point, boiling point, and conductivity
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Evaluate the energy conversion efficiency of a variety of energy transformations
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs the following items to make a heated water storage tank:
- Water container large enough to hold at least 1 liter (.22 gal), such as a plastic container (easier to cut) or coffee can
- Cardboard box, sized so it is about 1-inch (2.5 cm) bigger than the water container on all sides
- Insulation material (such as Styrofoam pellets, cotton or crumpled newspaper)
- 2 feet (61 cm) of plastic tubing (3/8-inch inside diameter, ½-inch outside diameter)
Each group needs the following items to make a solar collector:
- Cardboard box with transparent cover (4-inch deep box, ~12 x 12-inch, or 30 x 30 cm) with a transparent cover (sized to match box area dimensions) made from rigid clear plastic, saran wrap or other thin and transparent plastic material) or a disposable baking sheet pan, 9-inches x 13-inches x 2-inches deep (23 x 33 x 5 cm), that comes with a fitted clear plastic cover
- Insulation material (such as Styrofoam pellets, cotton or crumpled newspaper)
- Cardboard piece, same size as floor area of cardboard box or sheet pan
- 3 feet (91 cm) soft copper tubing (3/8-inch outside diameter; available at hardware stores)
Each group needs:
- Stopwatch (or a watch or timer to keep track of the passage of time up to 20 minutes)
- 4 Styrofoam cups (any size, but matching sizes so easy to double up for insulating purposes)
- Solar Water Heater Design and Analysis Worksheet, one per team
For the entire class to share:
- Scissors, to cut plastic and cardboard
- Packing or duct tape, and stapler, to make/modify cardboard boxes
- Drill (optional), to make a hole in a metal container; bit sized to create hole for plastic tubing
- Waterproof glue, such as epoxy or gorilla glue
- Aluminum foil, to line inside walls and base of solar collector
- Black spray paint
- String or twine, to tape to coiled copper tubing to help with measuring
- Copper tubing bender tool (see Figure 4; if not available, have students use a rigid cylindrical surface to bend the tubing around)
- Copper tubing cutter (see Figure 4; inexpensive and available at hardware stores) or a hack saw (cuts are not as clean)
- Hammer (optional), to hammer out kinks in the copper tubing
- Jug, to carry water outside
- 1 liter measuring cup
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_housing_lesson01_activity1] to print or download.Pre-Req Knowledge
Students should have a basic understanding of the three types of heat transfer (conduction, convection, radiation) as discussed in the associated lesson. Students should concurrently be taking Algebra 1 in order to complete the worksheet calculations.
Introduction/Motivation
When you hop into a hot shower, you probably don't think about the process the water went through before it came out of the shower head. Likewise, you might not consider the gas or electricity that was used to cook your dinner. In homes today, most of the methods used to heat water, to warm or cool your living room, or to cook food, involve the burning of fossil fuels (typically natural gas or oil), which not only costs money and is limited in its supply, but contributes to the emission of greenhouse gasses into our atmosphere. So how do we fix this problem, or at least make an improvement? The answer is right above us — the sun. In fact, already more than 10,000 U.S. homes use only energy generated from the sun's radiation.
How can we use energy from the sun to replace energy we would usually acquire by the combustion of fossil fuels? One way is to use something called a photovoltaic cell (found in solar panels) to generate electricity from the sun's thermal energy. This method is increasingly popular as it becomes more affordable. Another way is to use a solar water heating device that converts radiation from the sun into thermal energy that is transferred to water. This method often works in conjunction with a home's regular water heater to minimize the cost of heating water and reduce the effects on the environment. While solar water heating devices can be extremely expensive, their basic design is simple enough to make using common materials found at hardware stores. As our fossil fuel energy supply dwindles and becomes more costly, more homes are relying on solar hot water heaters as a way to shift towards using a cleaner energy source. It is one way to create more energy-efficient housing.
If you want to create an environmentally-friendly house, it is a smart idea to create a device that can extract solar energy from the sun and convert it into thermal energy to heat water for use throughout your house. Since up to 25% of a typical home's energy bill goes to heating water, homeowners can save money each year by using sunshine rather than electricity or natural gas. In fact, even a system that costs $7,000 to install could pay for itself in less than five years! Think of the impact if solar energy devices were incorporated into more of the housing in our world.
Procedure
Background
How solar water heating systems work: Solar water heaters function through the combination of two parts — a storage tank and a collector. The storage tank is a well-insulated container that stores the circulating water. The water travels to the solar collector, which is usually located on a rooftop. The collector consists of either a long coiled copper pipe through which water flows (Example A) or a series of parallel pipes across which water flows (perpendicularly) (Example B). Radiation energy from the sun is absorbed within the collector, and travels through the pipe via conduction. After the water has passed through the collector and absorbed heat, it returns to the storage tank and increases the temperature of the remaining water via convection. This process repeats until the water in the tank is sufficiently hot so that it may be used as domestic hot water (for showers and baths). Solar water heaters usually work in conjunction with an electric- or gas-powered water heating system, which kicks in if the solar water heater alone cannot meet demand.
Reasons for the use of certain materials: The copper pipe and bottom of the collector are painted black to improve their ability to absorb thermal energy. Black reflects the least amount of radiation (about 3%) of any color, and therefore absorbs almost all the radiation energy to which it is exposed.
Because of its high thermal conductivity, it is advantageous to use copper in the collector. Besides being relatively inexpensive and readily available, copper is among the best heat conductors, thus it transfers heat to water the quickest and most efficiently.
The solar collector is enclosed with a glass or protective transparent cover to trap radiant heat inside, which maximizes heating of the water passing through. The foil around the sides reflects more radiant energy into the absorbing components of the collector.
Practicality of solar water heating: The solar water heating method does not work effectively at night or when sunlight is blocked. Because of this, the water heater system still requires an auxiliary heater within the house. Also, solar water heaters work most effectively in climates with year round abundant sunshine and clear skies. While the solar water heater does not eliminate the need for an auxiliary water heater, it significantly reduces the cost of utilities by directly reducing the consumption of electricity or gas. The most common water heaters in homes today operate on electricity and natural gas. Note that while the use of electricity may seem not to harm the environment, one must consider the method used to generate the electricity in the first place (usually by burning coal or other fossil fuels at an electricity-generating plant).
Specific heat capacity of water: The specific heat capacity of water is the amount of heat required to raise the temperature of 1 gram (1 mL) of water by one degree Celsius. For water, this value is:
Heat transfer within the system: We can measure the solar energy absorbed by the system by measuring the heat gain of the water. We relate these two by saying:
where Qgained refers to the heat gained by the water, and Qlost refers to the amount of heat lost to the environment (equal to the solar energy absorbed by the system from the sun).
To calculate the heat gained by the water, we use the equation:
Where m is the mass of the water in grams, Cw is the specific heat of water (given previously), and ∆T is the change in temperature of the water in oC or K.
Example: Given a mass of water of 1 kg circulating through a heat exchanger, an initial temperature of 23oC and a final temperature of 25.5oC, calculate the heat transfer from the exchanger to the water. Assume an adiabatic system.
Activity Schedule: Student teams design, create and test their own solar water heater devices according to the following activity stages.
- Day 1 (60 minutes) design and create water containers (tanks)
- Days 2 and 3: (120 minutes) design and create solar collectors, and attach them to the water tanks
- Day 4 (60 minutes) test and evaluate the solar water heater devices outside on a sunny day
Before the Activity
- Ask students to bring any supplies that are readily available from home (such as plastic or metal water containers, cardboard boxes, insulation materials, plastic covers, saran wrap, aluminum foil, watches, Styrofoam cups, etc.).
- Gather materials.
- Prepare solar water heater examples to show to the students. This might be photographs, or a solar water heater made in advance by the teacher, or old student examples, or an actual solar water heater taken apart.
- Make copies of the Solar Water Heater Design and Analysis Worksheet, one per team.
Day 1: Design/Build Water Containers (60 minutes)
Accomplish the following during Day 1:
- Divide the class into groups of two or three students each.
- Give each group a copy of the four-page Solar Water Heater Design and Analysis Worksheet.
- Explain how the finished product might work, and show examples.
- Direct student teams to design their water heater within the constraints of the provided materials (see Part 1 on worksheet). As they work through this stage, provide guidelines and suggestions to help them out, as needed.
- Have students gather materials.
- Have students create their water storage container (tank) according to the Part 1 procedure described below.
Part 1: Water Storage Container Design
- 1. Use cardboard, scissors and strong tape to make a box to house the water container, or use/modify an existing box of the right/similar size. Leave at least 1 inch (2.5 cm) on each side for insulation (see Figure 1).

- Cut or drill a hole that matches the diameter of the ½-inch plastic tubing near the bottom of the water container.
- Fit the container into the box and cut a hole in the box, also the same diameter as the plastic tubing, at the point at which the water container hole lines up.
- Insert plastic tubing into the hole in the box and water container (see Figure 2). Seal any gaps around the tubing with waterproof glue to prevent leaking.

- Fill the area between the box and the water container with insulating material (see Figure 2).
Days 2 and 3: Design/Build Solar Collectors (120 minutes)
Accomplish the following during Days 2 and 3:
- Have student teams design and build a solar collector as described in the Part 2 procedure below.
- Direct them to use one of the two copper tubing designs provided (described under Example A and Example B), or create their own. Creating a collector with a long coiled copper pipe through which water flows (Example A) works in a cardboard box collector. Creating a collector with a series of parallel pipes across which water flows perpendicularly (Example B) requires a waterproof container such as a disposable baking sheet pan with a fitted clear cover.
- As part of the design process, encourage students to find a way to bend their copper tubing (around something circular) and explain why using just their arms does not work (described below).
- Final assembly. Have students attach their water storage container to their collectors, according to the Part 3 procedure described below.
Part 2: Collector Design
- Use cardboard to create a box (or use/modify an existing box) about 4-inches deep that matches the size of your transparent plastic cover sheet. In this approach, water flows through the copper tubes, so a water tight collector is not necessary (cardboard box works fine).
- Line the inside walls of the box with aluminum foil, shiny side out (see Figure 3).

- Layer the bottom of the box with about 1 inch (2.5 cm) of insulating material.
- Cut a piece of cardboard to fit the inside floor of the box. Cover it with aluminum foil and paint it black, then place it over the insulated floor of the box (see Figure 3).
- Direct student teams to determine their coil design. Two examples, A and B, are described below, copper bent into curves and copper in straight pieces. Or, student teams can create their own design.
- Guide students as they measure, bend and cut the soft copper tubing allotted for their team. Share the following tips to help them work with the copper:
- Measuring the copper tubing: Copper tubing usually comes in a coil (see Figure 4). To measure the length of it accurately, use a premeasured piece of string (such as 12 inches) as a flexible ruler. Tape it along the coiled tubing as demonstrated in Figure 4.
- Bending the copper tubing: To bend the tubing, use a copper bending tool (see Figure 4), or a rigid curved surface that has the desired radius. To make straight pieces, use the bender to remove the slight curve of the coiled copper. Note: The copper cannot be bent just using your arm strength because you will not get the desired radius, and you will probably kink the tubing. If the pipe gets kinked, you can partially fix it by hammering it back into shape.
- Cutting the copper tubing: Use a copper tubing cutter (shown in Figure 4), or a hack saw.

Coil Design - Example A:
- For one design approach, bend the copper tubing into a continuous S-shape. Figure 5 provides an example with dimensions; modify the measurements and shape according to the team's design preference.
- Use spray paint to paint the copper tubing black.
- Cut two holes in the box that align with the points at which the copper tubing comes out.
- Insert the copper tubing into the box and secure it with glue (see Figure 5).
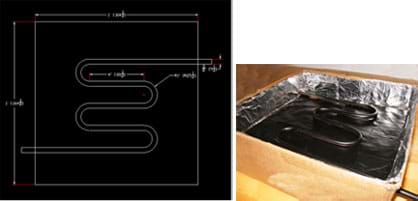
- Cover the box with its plastic sheet cover, and seal the edges.
Coil Design - Example B
- For another design approach, cut three or four 8-inch (20.3-cm) pieces of straightened copper tubing and glue them to the bottom of a disposable baking pan. Paint the entire bottom black (see Figure 6). In this approach, water flows over the tubes so the collector must be water tight.
- Cut holes in both of the shorter sides of the baking pan (see Figure 6). Make the hole diameters the same size as the copper tubing.
- Insert two pieces of short, straight copper tubing (½-inch to 1-inch length, or 2.5 to 5 cm) extending out of each hole in the pan (see Figure 6).
- Cover and secure the box with its transparent cover.

Part 3: Assembling the System
Attach the plastic tubing to the end of the copper tubing. Seal the connection with waterproof glue (See Figure 7 for finished product).

Day 4: Test Solar Water Heaters (60 minutes)
Accomplish the following during Day 4:
- On a sunny day (it is okay if it is cold, but it must be sunny), bring the class outside to test their model solar water heater devices. Bring timers, worksheets, jug of water, measuring cup and Styrofoam cups.
- Have students organize within their teams and assign roles (circulate water through the system [collect and pour], timer, measure temperature, record measurements, etc.).
- Have students follow the experimental test procedure steps described in the worksheet.
- Once all groups have recorded their data, bring the class back inside.
- Have students complete the calculations and evaluation steps of the worksheet. Encourage them to work as a team and discuss each question before agreeing upon their answer.
- Once students have completed all four pages of the worksheet, lead a class discussion to review their findings and explore re-design (improvement) ideas. For details, see the post-activity assessment in the Assessment section.
Vocabulary/Definitions
adiabatic: Occurring without gain or loss of heat, such as a process in which no heat transfers between the system and its surroundings.
combustion: The process of converting fuel into heat.
conduction: The transfer of heat through a substance by direct contact of atoms or molecules.
convection: The transfer of heat by circulation of a gas (such as air) or liquid (such as water).
fossil fuels: Carbon-based sources of energy such as coal, oil and natural gas. These are not renewable sources of energy.
heat transfer: The flow of heat from one substance to another. Heat always transfers from a higher temperature to a lower temperature.
insulation: A material of low thermal conductivity used to reduce heat loss.
model: (noun) A standard or example for imitation, comparison or analysis. (verb) To simulate, make or construct something to help visualize or learn about something else (as a product, process or system) that cannot be directly observed or experimented upon, often at a smaller scale.
radiation: Heat radiated in the form of rays or waves (such as rays from the sun).
renewable energy: Energy generated from resources that are unlimited, rapidly replenished or naturally renewable such as wind or solar energy.
solar energy: Energy derived from the sun in the form of solar radiation.
specific heat capacity: The amount of heat that must be added or removed from a unit mass of that substance to change its temperature by one degree.
thermal conductivity: Measure of the ability of a solid or liquid to transfer heat.
thermal energy: Kinetic energy due to the motion of particles and atoms within a substance.
Assessment
Pre-Activity Assessment
Question/Answer: Write the following information on the board. Ask the students and discuss as a class:
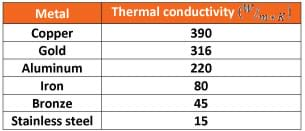
- Looking at this table of conductivity values for certain metals, what is the reason for choosing copper tubing for a heating device? (Answer: It has the highest conductivity value and thus transfers heat the fastest.)
- What is the purpose of insulating a water tank? (Answer: To prevent the transfer of heat out of the tank and back into the atmosphere [improve efficiency, achieve optimization].)
- What is the point of painting the copper tubing black in a heating device? (Answer: Black absorbs more solar energy than any other color, reflecting only 3% of the sunlight that hits it. By using black, we help more heat to be absorbed by the water.)
Discussion: Hold a class discussion prior to the start of the activity about criteria that may be important in designing a solar water heater and lead the students to prioritize the constraints.
Activity Embedded Assessment
Worksheet: Have student teams use and complete the activity worksheet; review their answers to gauge their mastery of the subject.
Post Activity Assessment
Group Discussion: Ask students and discuss either as a class and/or amongst teams:
- Can all three types of heat transfer be observed in the finished product? If so, where? (Answer: Yes, the system absorbs heat through radiation from the sun, the heat is then conducted through the copper piping and to the water, and the warm water transfers its heat to the cooler water in the tank by convection.)
- What are the pros and cons of using this type of heating system for your home water heating needs? (Possible answers: Pros — Uses a renewable energy source, reduces the use of energy from fossil fuel sources, reduces emission of harmful chemical byproducts to the environment, and reduces the cost of your utility bill. Cons — Cannot collect the sun's energy during the night and during times when cloud cover blocks the sun, which is much of the year in some locations; requires up front cost to install the system; cannot completely replace another type of system.)
Engineering Re-Design Queries: Have students think about their water heater design. What worked? What didn't work? What is the reasoning behind the way you built your water heater? Could you have built it a better way? What if you had unlimited materials or different time constraints? Have students think about the trade-offs involved in their design. If time allows, or for homework, have students make a sketch or write a description about design changes they would make and why.
Troubleshooting Tips
If you have trouble bending the copper tubing, try using a larger radius rigid guide, such as a sturdy cylindrical pipe or another suitable object found around the school. It sometimes helps to have extra tubing to serve as a longer lever to pull on when bending the copper (to increase the torque exerted).
If your copper tubing gets a kink in it, hammer it out on a hard surface, and then straighten it out again.
Activity Extensions
Have students come up with some ways to improve the overall efficiency of their device based on their observations and assuming no constraints on materials.
Have students improve their design by modifying it, using a new material, or swapping out a material (if possible).
Activity Scaling
- For younger students, instead of building a water heater, bring in some sort of heat exchanger and let them measure its heat transfer.
- For more advanced students, incorporate a simple pumping system (such as a small aquarium pump) into the water heater so the water can circulate on its own much as it would in a large-scale water heating system.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn and discuss the advantages and disadvantages of renewable and non-renewable energy sources. They also learn about our nation's electric power grid and what it means for a residential home to be "off the grid."

Students learn about the nature of thermal energy, temperature and how materials store thermal energy. They discuss the difference between conduction, convection and radiation of thermal energy, and complete activities in which they investigate the difference between temperature, thermal energy and ...

Students calculate the amount of solar energy available at a given location and time of day on Earth. They learn the importance of determining incoming solar energy for solar devices.

Students are introduced to passive solar design for buildings — an approach that uses the sun's energy and the surrounding climate to provide natural heating and cooling. They learn about some of the disadvantages of conventional heating and cooling and how engineers incorporate passive solar design...
References
Kurtus, Ron. Ways Heat Is Transferred – Succeed in Physical Science. Revised May 26, 2006. School for Champions LLC. www.school-for-champions.com/science/heat_transfer.htm Accessed February 6, 2008.
List of Thermal Conductivities. Wikipedia. Updated February 2, 2008. en.wikipedia.org/wiki/Thermal_conductivity#List_of_thermal_conductivities Accessed February 6, 2008.
Solar Energy. Montgomery County, MD, Department of Environmental Protection. www.montgomerycountymd.gov/dectmpl.asp?url=/content/dep/energy/CleanEnergySolar.asp Accessed February 5, 2008.
Solar Hot Water. Wikipedia. Last modified February 5, 2008. www.wikipedia.org Accessed February 6, 2008.
Other Related Information
This activity was selected to be part of the Climate Literacy & Energy Network (CLEAN) reviewed and annotated digital library collection. CLEAN, an NSF National Science Digital Library (NSDL) Pathway, seeks out exemplary digital resources that relate to key climate and energy concepts, are scientifically robust and current, and are easily accessible online. The focus is on strong learning activities, with solid pedagogical scaffolding for grades 6-16. This resource passed a rigorous peer-review process as part of being selected to be included in the collection in April 2011. For the complete review, see: cleanet.org/resources/41891.html
Copyright
© 2007 by Regents of the University of Colorado.Contributors
Landon B. Gennetten; Lauren Cooper; Malinda Schaefer Zarske; Denise W. CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: January 6, 2021





User Comments & Tips