Quick Look
Grade Level: 7 (5-7)
Time Required: 2 hours
(two 60-minute sessions)
Expendable Cost/Group: US $10.00 This activity also requires the use of non-expendable (reusable) LEGO MINDSTORMS NXT robot kits, temperature sensors and software; see the Materials List for details.
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Measurement
NGSS Performance Expectations:

| MS-ETS1-3 |
| MS-PS1-2 |
| MS-PS1-4 |

Summary
Students learn that fats found in the foods we eat are not all the same; they discover that physical properties of materials are related to their chemical structures. Provided with several samples of commonly used fats with different chemical properties (olive oil, vegetable oil, shortening, animal fat and butter), student groups build and use simple LEGO® MINDSTORMS® NXT robots with temperature and light sensors to determine the melting points of the fat samples. Because of their different chemical structures, these fats exhibit different physical properties, such as melting point and color. This activity uses the fact that fats are opaque when solid and translucent when liquid to determine the melting point of each sample upon being heated. Students heat the samples, and use the robot to determine when samples are melted. They analyze plots of their collected data to compare melting points of the oil samples to look for trends. Discrepancies are correlated to differences in the chemical structure and composition of the fats.Engineering Connection
Understanding the chemistry of fats, as well as compounds that contain fat, is important to chemical and biomedical engineers. Fat molecules are incorporated into cosmetics, medications and food, as well as found in human blood. When designing certain products, engineers take into consideration various fat types and their physical properties. For example, chemical engineers who design skin creams or makeup must know what type of fats to put in products to give them the desired solid or liquid consistency. In the food processing industry, chemical engineers must understand the various properties of different fats so they know their potential effects on food products and human digestion. Some biomedical engineers design devices that interact with blood, such as stents and pacemakers, which are implanted into arteries or valves and must be resistant to the deposition of fats on their surfaces. Engineers who design new medications and drugs also need to know how they will react with fats, which are present in human cells and blood. For all these applications, engineers need to have a clear understanding of the chemical and physical properties of fats.
Learning Objectives
After this activity, students should be able to:
- Construct a LEGO NXT robot to perform a specific type of measurement.
- Explain the functions of light and temperature sensors.
- Define the term "melting point" and explain its relationship to the chemical composition of fats.
- Explain and infer how certain properties, such as melting point, can be determined through the measurement of other properties, such as translucency, of a material.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-3. Analyze data from tests to determine similarities and differences among several design solutions to identify the best characteristics of each that can be combined into a new solution to better meet the criteria for success. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze and interpret data to determine similarities and differences in findings. Alignment agreement: | There are systematic processes for evaluating solutions with respect to how well they meet the criteria and constraints of a problem. Alignment agreement: Sometimes parts of different solutions can be combined to create a solution that is better than any of its predecessors.Alignment agreement: Although one design may not perform the best across all tests, identifying the characteristics of the design that performed the best in each test can provide useful information for the redesign process—that is, some of the characteristics may be incorporated into the new design.Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze and interpret data to determine similarities and differences in findings. Alignment agreement: Science knowledge is based upon logical and conceptual connections between evidence and explanations.Alignment agreement: | Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Alignment agreement: Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to predict and/or describe phenomena. Alignment agreement: | Gases and liquids are made of molecules or inert atoms that are moving about relative to each other. Alignment agreement: In a liquid, the molecules are constantly in contact with others; in a gas, they are widely spaced except when they happen to collide. In a solid, atoms are closely spaced and may vibrate in position but do not change relative locations.Alignment agreement: The changes of state that occur with variations in temperature or pressure can be described and predicted using these models of matter.Alignment agreement: The term "heat" as used in everyday language refers both to thermal energy (the motion of atoms or molecules within a substance) and the transfer of that thermal energy from one object to another. In science, heat is used only for this second meaning; it refers to the energy transferred due to the temperature difference between two objects.Alignment agreement: The temperature of a system is proportional to the average internal kinetic energy and potential energy per atom or molecule (whichever is the appropriate building block for the system's material). The details of that relationship depend on the type of atom or molecule and the interactions among the atoms in the material. Temperature is not a direct measure of a system's total thermal energy. The total thermal energy (sometimes called the total internal energy) of a system depends jointly on the temperature, the total number of atoms in the system, and the state of the material.Alignment agreement: | Cause and effect relationships may be used to predict phenomena in natural or designed systems. Alignment agreement: |
Common Core State Standards - Math
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
-
Construct and interpret scatter plots for bivariate measurement data to investigate patterns of association between two quantities. Describe patterns such as clustering, outliers, positive or negative association, linear association, and nonlinear association.
(Grade
8)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Interpret the accuracy of information collected.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
New York - Science
-
Develop a model that predicts and describes changes in particle motion, temperature, and phase (state) of a substance when thermal energy is added or removed.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Analyze data from tests to determine similarities and differences among several design solutions to identify the best characteristics of each that can be combined into a new solution to better meet the criteria for success.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- LEGO MINDSTORMS NXT robot, such as the NXT Base Set (5003402) for $159.98 at https://shop.education.lego.com/legoed/en-US/catalog/product.jsp?productId=5003402& isSimpleSearch=false&ProductLine=NXT
- LEGO MINDSTORMS Education NXT Software 2.1, available as a single license (2000080) for $39.97 or a site license (5003413) for $271.96 at https://shop.education.lego.com/legoed/en-US/catalog/product.jsp?productId=prod120017&isSimpleSearch=false&ProductLine=LEGO+MINDSTORMS+Education+NXT
- computer, loaded with NXT 2.1 software
- LEGO NXT temperature sensors, for $27.96 each at https://shop.education.lego.com/legoed/education/NXT/NXT+Temperature+Sensor/9749&isSimpleSearch=false
- 9 samples of various fats with different chemical structures (approximately 5 ml or 5 g/group):
- olive oil
- butter
- animal lard
- vegetable shortening
- frying fat
- corn oil
- canola oil
- soybean oil
- peanut oil
- 9 glass test tubes
- Bunsen burner, flame and gas source
- small beaker filled with water
- a stand, to support the beaker of water over the Bunsen burner
- container filled with ice to chill the samples
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/nyu_fat_activity1] to print or download.Pre-Req Knowledge
Prior experience with data collection, data plotting, and safe and correct Bunsen burner use.
Introduction/Motivation
For all the materials in our physical world, the physical properties (the ones we can see and touch) are defined by the chemical properties of the materials. These chemical properties are invisible to the human eye. How, then, can we get an understanding of the chemical properties if we cannot see or feel them? On the other hand, the physical properties affect how we interact with these materials, including how our bodies digest food!
You probably eat a wide range of foods in your diet. What you may not know is that different types of fats are present in the foods that you eat. All fats are not the same. Some fats are better for you than others, and this is based on what the fat molecules in those foods look like! Since fat molecules are too small to see with our eyes, we can use sensors and robots to get a better understanding of how different fats react when they are heated. As you know, all matter (even food!) is found in one of three states: solid, liquid or gas. The melting point is the temperature at which a material turns from solid to liquid. By measuring the melting point of different fat samples, we can get an idea of how easily each type of fat is broken down in our bodies, and therefore, how good or bad it might be for us.
Understanding how chemical properties are related to physical ones is very important for chemical and biomedical engineers. These engineers often work with materials that contain fats, such as cosmetics (like makeup or creams), biological materials (like blood and cells), and of course food. They use their knowledge of the chemistry of fats to design medical devices that can treat patients (and even save lives) or to devise new types of foods. One of the most important properties of fats that engineers need to know is their melting points.
Procedure
Background
Students determine the melting points of samples of edible fats and explore how that information affects their own diet and health. Fats are an important class of nutrients found in food. They occur naturally in plants and animals, and are incredibly efficient in storing energy necessary for biochemical reactions in organisms. The building blocks that make up fats are called fatty acids. One type of fatty acid, called linoleic acid, is essential in human nutrition in small amounts. Unfortunately, many people consume too much unhealthy fat, which can lead to serious health problems. Lipids (fats) containing trans fats have been shown to be especially detrimental to human health; their chemical structure leads to melting points above the temperature of the human body. This property makes it difficult for our bodies to process them, causing trans fats to remain solid in our gastrointestinal tracts and bloodstreams. Fats with higher trans fat content exhibit higher melting points, making them more dangerous to human health.
Food is needed to keep living things alive and certain foods are better for nourishing bodies than others. To do systematic analyses of food, it is often broken down into several nutritional categories. Fat is one of these groups, whch can be further divided into subgroups including: saturated fat, monounsaturated fat, polyunsaturated fat, and trans fat. Not all fat that humans ingest is the same, either chemically or physically.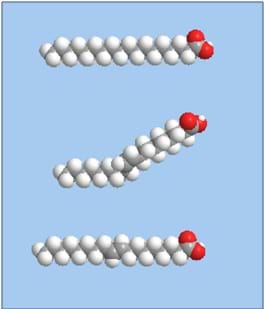
The reason for the discrepancy in melting points exhibited by different types of fat is due to the structure of the fatty acid chains that make up fats and oils. Figure 1 shows that different types of fat have different chemical structures. Note that trans fat and saturated fat are straight, while monounsaturated fat is bent. This leads to differential packing of the molecules in fats, in which straight molecules are able to pack tightly next to one another. Bent molecules have more disorganized packing, making them easier to break apart from one another. This is the reason they have different melting points: less energy is required to break apart bent molecules than closely packed straight molecules. Trans fats have been documented to have melting points in the range of 45 °C (113 °F), while monounsaturated fats have melting points in the range of 13 °C (55.4 °F). With the temperature of the human body being 37 °C (98.6 °F), trans fats remain solid in the body, leading to an array of complicated health problems when consumed often enough.
Before the Activity
- Prepare the oil and fat samples. For each one, pour approximately 5 ml into a test tube. Heat up solid fats such as butter and lard to make it possible to pour samples into test tubes. In total, prepare nine samples (per group): olive oil, butter, animal lard, vegetable shortening, frying fat, corn oil, canola oil, soybean oil, peanut oil.
- Store the samples in a refrigerator or on ice until used.
- Open the program entitled Heat Fat with the LEGO MINDSTORMS Education NXT software. Download the program onto each robot. Explain the function of the program to students, using the description below and the program schematic in Figure 2 as a guide.
Function of the program: The main function of the program is to indicate exactly when a sample has melted. It does this by continuously testing the reflected light value until it falls below the threshold, indicating that the light is no longer being reflected (as with an opaque solid sample), but transmitted (through a liquid translucent sample). When the light is being transmitted, the program checks the temperature via the temperature sensor and then displays the numerical temperature value on the display screen. 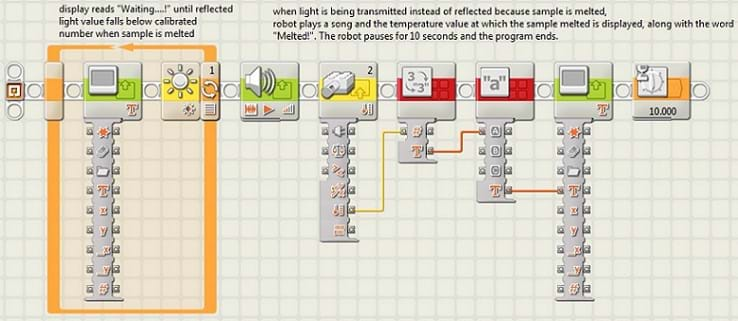
- In front of the class, demonstrate the measurement process using the robot set-up shown in Figure 3.
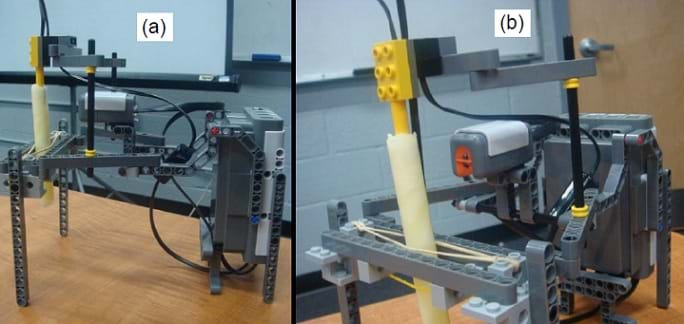
Figure 3. Two views of the LEGO NXT robot set-up to measure reflected light and temperature of a fat sample. - Make copies of the Fat Chemistry Worksheet, one per student.
- Divide the class into groups of approximately 5 students each. Supply each group with a LEGO kit and the nine fat samples.
With the Students
- Give a brief lecture on how the chemistry of fats is related to the physical properties. Explain that when a fat is solid, it is opaque, and when it melts and changes to a liquid state, it becomes translucent. This physical characteristic its translucency, can be used to determine when a sample has melted.
- Use the Fat Chemistry Presentation to present background information.
- Have groups design their own robots with both temperature and light sensors to be used for measuring the melting point of fat samples.
- Design a robot: For this activity, each student team designs and physically constructs a robot that is able to sense both reflected light and temperature of a sample. This robot must have a light sensor and a temperature sensor to be able to perform this task. Use LEGO pieces to build a holder that positions the light sensor at a constant distance from the test tube while measuring each sample (see Figure 3 for an example). Use cables provided in the LEGO kit to connect the light sensor to the brick via port 1 and the temperature sensor via port 2.
- Note: Students are not responsible for writing the LEGO MINDSTORMS NXT program for the robot. Have them use the attached program Heat Fat to perform this activity.
- Assist groups in building their robots to meet the necessary experiment set-up.
- Each group should have a Bunsen burner for heating a water bath containing the sample. While Bunsen burners are in use, carefully supervise groups for safety.
- Groups test one sample at a time, slowly heating the sample until the robot indicates that the sample has reached its melting point. Each group member records the melting point on his or her worksheet. This process is repeated for each of the nine samples.
- Direct students to plot the melting points of the samples vs. the saturated/trans fat content (in weight percent) on their worksheets (use the blank graph with pre-labeled axes).
- Give students time to summarize their results and describe what they learned about edible fats on the worksheet.
Vocabulary/Definitions
fatty acid: The building blocks that make up naturally occurring fats, such as fats and fatty oils from animals and plants.
lipid: A class of molecules that are insoluble in water, including fats, grease and oils. They are the principal structural component of living cells.
melting point: The temperature at which a solid melts and becomes a liquid.
monounsaturated: A type of fatty acid molecule that contains one double bond, and has two fewer hydrogen atoms than a saturated fat.
opaque: Blocking the passage of light.
phase change: The change of physical phase of a material: from solid to liquid, liquid to gas, or the reverse of either of those processes.
polyunsaturated fat: A fatty acid molecule that contains more than one double bond, and has a reduced number of hydrogen atoms.
saturated fat: Fats in which all carbon atoms are bonded to hydrogen atoms and contain no double bonds between the carbons.
temperature: The degree of hotness of coldness measured on a definite scale.
trans fat: A commercially produced form of monounsaturated fats, in which the carbon chains on either side of the double bond are arranged opposite to one another. These fats are not naturally occurring.
translucent: Transmitting light through an object.
Assessment
Pre-Activity Assessment
Class Survey: Before beginning the activity, administer the Pre-Survey on Fat Chemistry to assess students' prior knowledge. Do students think all fats they eat are the same? What types of engineers might need to understand chemistry of fats and foods? Ask students the following preliminary questions:
- Is fat good for us? (Answer: Yes, fat is necessary for humans to stay healthy. Certain, good-quality fats are best for our bodies. Certain fats are easier for our bodies to process, meaning that they probably won't stay in our cells long and can keep us healthier throughout our lives.)
- Is oil considered a fat? (Answer: Yes, oil is considered a fat.)
- What type of engineer might work with chemistry, or the chemistry of food or fats? (Answer: Chemical and biomedical engineers need to understand the complex chemistry of fats. Fat molecules are used in many products such as cosmetics and medications, and are also found in blood. Chemical engineers design products taking into consideration what types of fats, and their physical properties, can be useful. Biomedical engineers design devices such as stents and pacemakers that are implanted into the body and must be resistant to deposition of fats on their surfaces.)
- What type of engineer would work with robots, and why? (Answer: The work of all types of engineers can benefit from using robots. Robots can help chemical engineers perform tests on the properties of chemicals and control the generation of new products. Tiny robots can also be used by biomedical engineers to access remote parts of the human body that doctors have trouble reaching to provide treatment. Mechanical and electrical engineers are commonly responsible for design and programming of robots for all types of applications.)
- What happens when we heat up fat? (Answer: When fat is heated up, the molecules move around slightly within the fat, leading them to disconnect from one another. This can turn a solid fat into a liquid, or make a liquid fat less viscous, which means it flows easier.)
- What are some examples of fat? Name some physical properties of fats that might differ. (Answer: Some examples of fat include: oils, butter and animal fat [lard]. Some physical properties of fats that might differ depending on the type are color, texture, state at room temperature [liquid vs. solid], smell and viscosity.)
Activity Embedded Assessment
Worksheet: During the activity, have students use and complete the Fat Chemistry Worksheet by collecting data, recording observations, graphing results and answering a series of questions related to fat chemistry and data analysis. For the trans fat samples, students plot the temperature vs. percent fat composition and determine the trend. They provide their synthesis of this trend in one of the worksheet questions.
Post-Activity Assessment
Concluding Class Survey: Have students complete the Post-Survey on Fat Chemistry as a detailed assessment on the concepts presented in this activity. Compare to the pre-survey results to gauge how much students learned during the activity.
Safety Issues
- Students with long hair must tie it back when working near the Bunsen burners.
- Inform students of the correct use of Bunsen burners; Be sure to turn off the gas source when they are not in use.
- Supervise students at all times when using Bunsen burners.
- Do not fill test tubes to the top with fat samples, as some of the sample can drip out and burn while being heated, which might lead to a grease fire.
Troubleshooting Tips
Make sure that the robots are stable so that the set-up does not fall during the experiment.
In advance of the activity, calibrate the light sensor values in order to accurately determine when each sample is melted.
Activity Extensions
Develop your own model: The LEGO MINDSTORMS model used in this activity uses reflected light as a measurement melting point. Have the students brainstorm with a partner and develop a design of a new model that uses a different mechanism to measure melting point. Depending on the level of students, "research time" could be included in the brainstorm portion so that students could research the different ways scientists and engineers typically measure the melting point of various substances.
Activity Scaling
- For lower grades, simply record temperature values and discuss the results, skipping the graph/data plotting.
- For upper grades, record temperature values and use composition data found in nutritional information labels to calculate the percent composition of saturated/trans fat. Plot the melting temperature vs. the percent composition saturated/trans fat.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn how food packages are designed and made, including three main functions. Then, in the associated activity, students act as if they are packaging engineers by designing and creating their own food packages for particular food types.

This lesson introduces students to the main parts of the digestive system and how they interact. In addition, students learn about some of the challenges astronauts face when eating in outer space. Engineers figure out how to deal with such challenges.

Students are introduced to chemical engineering and learn about its many different applications. An associated hands-on activity gives students a chance to test their knowledge of the states of matter and how to make observations using their five senses: touch, smell, sound, sight and taste.
References
Lodish, Harvey et al. Molecular Cell Biology. 4th edition. W. H. Freeman and Company.
Investigation 1: The Fat Test. Life Science, Food and Nutrition. Full Option Science System. Delta Education.
Vegetable Oils Summary: Cooking Oil Characteristics. Last updated July 21, 2012. Vaughn's Summaries, 2005 Vaughn Aubuchon. Accessed February 13, 2013. http://www.vaughns-1-pagers.com/food/vegetable-oils.htm
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 Polytechnic Institute of New York UniversityContributors
Jasmin HumeSupporting Program
AMPS GK-12 Program, Polytechnic Institute of New York UniversityAcknowledgements
This activity was developed by the Applying Mechatronics to Promote Science (AMPS) Program funded by National Science Foundation GK-12 grant no. 0741714. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: August 4, 2017





User Comments & Tips