Quick Look
Grade Level: 10 (9-12)
Time Required: 45 minutes
Expendable Cost/Group: US $18.00 This activity also uses some non-expendable (reusable) lab equipment; see the Materials list for details.
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Physical Science, Science and Technology
NGSS Performance Expectations:

| HS-ETS1-2 |
Summary
Student teams learn how water filtration systems that use nanoparticles and nanotechnology can remove organic compounds from water. First they learn about the role nanoparticles play in water filtration. Then they are introduced to the basics of nanoparticles and nanotechnology, focusing on the impacts and benefits this innovative technology has on our daily lives. Using methylene blue and methyl orange solutions, students test for the efficiency of photocatalytic nanoparticles to sanitize water. They expose a solution sample of water and methyl orange (the microbe indicator) with their newly-made water sanitation filters under UV light (sunlight) to activate the photocatalytic properties of three specific nanoparticles. They visually compare them with control samples to determine the best photocatalytic nanoparticle to sanitize water.Engineering Connection
Water is vital for human life and yet the availability of clean drinking water is a major issue facing society today. Exciting advances in the field of nanotechnology now allow engineers to build new water filters that may eventually replace filters that are currently in use—particularly those that contain petrochemicals or are made of harmful toxins that can potentially leach out. Nanotechnology engineers design and create nanostructures and specific-target nanoparticles that can destroy unwanted organic compounds and organisms in our water.
Learning Objectives
After this activity, students should be able to:
- Explain how nanoparticles can have photocatalytic properties.
- Demonstrate the process of photocatalysis.
- Determine which nanoparticle more efficiently break down the dye by calculating the time when photocatalysis is complete.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed. Alignment agreement: | |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
know specific hazards of chemical substances such as flammability, corrosiveness, and radioactivity as summarized on the Material Safety Data Sheets (MSDS); and
(Grades
10 -
12)
More Details
Do you agree with this alignment?
-
demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials.
(Grades
10 -
12)
More Details
Do you agree with this alignment?
-
organize, analyze, evaluate, make inferences, and predict trends from data; and
(Grades
10 -
12)
More Details
Do you agree with this alignment?
-
develop and use general rules regarding solubility through investigations with aqueous solutions;
(Grades
10 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 8 clear plastic 2-oz. cups with lids
- methyl orange solution; 3 ml for each sample and 3 ml for the control (equivalent to 1 full pipette, approx. 12 ml); 30 ml for $4.50 from Home Science Tools
- methylene blue solution; 3 ml for each sample and 3 ml for the control (equivalent to 1 full pipette, approx. 12 ml); 30 ml for $4.50 from Home Science Tools
- titanium dioxide (TiO2) sample solution (prepared by teacher using 5 g TiO2); enough for both dye samples (3 drops = 1 sample); 100 g of TiO2 nanopowder for $64 from SkySpring Nanomaterials
- magnesium oxide (MgO) sample solution (prepared by teacher using 5 g MgO); enough for both dye samples (3 drops = 1 sample); 25-g MgO nanopowder for $43 from SkySpring Nanomaterials
- zinc oxide (ZnO) sample solution (prepared by teacher using 5 g ZnO); enough for both dye samples (3 drops = 1 sample); 100-g ZnO nanopowder for $50 from SkySpring Nanomaterials
- 220 ml distilled water
- stopwatch
- 6 plastic pipettes
- 50-ml graduated cylinder, to measure distilled water
- black permanent marker pen, to label samples
- safety equipment: lab apron, safety goggles, gloves
To share with the entire class:
- Photocatalysis Presentation, Microsoft® PowerPoint® file
- Titanium dioxide (TiO2) smart coat photocatalyst YouTube video
- computer with internet connection and projector, to show the slide presentation and online video
- access to outside; sunlight is the UV light necessary for photocatalytic reactions
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/rice2-2254-nanoparticles-photocatalytic-speed-filtration-system] to print or download.Introduction/Motivation
(See the Pre-Activity Assessment section for guiding questions. Expect the introduction and presentation to take a maximum of 25 minutes.)
Opening Questions: Survey students' knowledge about nanoparticles and nanotechnology by asking them the following questions before presenting the Photocatalysis Presentation.
- What are nanoparticles? (Listen to student ideas, definitions and suggestions.)
- What is nanotechnology? (Listen to student ideas, definitions and suggestions.)
Nanotechnology is the science, engineering and technology that occurs at the atomic, molecular, and supramolecular scales. Nanotechnology has opened a new world of innovative engineering opportunities that allow us to build structures and products on a molecular scale. Nanotechnology offers cleaner, safer, and smarter products in fields such as medicine, agriculture, and transportation. It can also help improve manufacturing processes and produce high-quality products at a very low cost.
Nanoparticles are small particles in the 1-100 nm size range. With these particles, engineers and scientists can develop new products and accomplish a range of chemical reactions at the molecular level.
Nanoparticles are a major advancement in science, mainly because these structures can be modified, doped (i.e., combined with other structures) and coupled as nanocomposites which change the properties and composition of the nanoparticle in specific and unique ways. For example, engineers and scientists have synthesized nanoparticles to work as biosensors for major cellular compounds like glucose, hemoglobin, and cancer cells. In genetic engineering, new nanostructures have been engineered to mimic large-scale molecules in essential biological processes.
- How do you think nanotechnology might be used in our natural water resources? (Listen to student ideas, definitions and suggestions.)
Nanoparticles with photocatalytic properties were developed by engineers in response to the many pollutants found in our water resources. Organic chemicals produced for both industry and domestic use must degrade before they are released into the environment. Naturally, UV sunlight initiates the breakdown and catalysis of these organic chemicals, resulting in carbon dioxide and other minerals as the final byproducts. The photocatalytic detoxification of wastewater is a process requiring catalysis with solar technologies. The application of photocatalytic nanoparticles has been successful for degrading undesirable organic compounds in semiconductor photocatalysis for a variety of compounds. Ultimately, the best photocatalyst is one that is inexpensive, non-toxic, stable and highly photoactive. Nanotechnologists have developed different ways to test and experiment with nanoparticles to improve our water quality through sanitation and purification processes with photocatalysis as the primary focus.
Present the Photocatalysis Presentation.
(Show the video on titanium dioxide nanoparticles below)
Titanium dioxide (TiO2) smart coat photocatalyst video:
Today, we will investigate different nanoparticles with photocatalytic properties to determine which nanoparticles bleach (or “photo-sanitize”) dyed water the fastest after being exposed to UV light.
Magnesium Oxide
Magnesium oxide nanoparticles are odorless and nontoxic, possessing high hardness, high purity and a high melting point. Magnesium oxide has a specific molecular structure of a crystalline lattice between the magnesium and oxygen ions that form a high electrostatic force within the compound (see Figure 1).
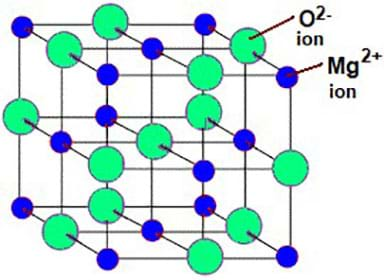
Magnesium oxide nanoparticles are used primarily as catalysts and scrubbers for air pollutant gases. They are also used as superconductors because of their unique multi-faced surface structure, which is often used to combine with other particles as a nanocomposite for an extra stability in its chemical and electrical properties. Applications of magnesium oxide include petrochemical production, soundproof and heat insulation, fire retardants, and different types of product coatings. Magnesium oxide is also used as an antibacterial agent to enhance food safety.
Zinc Oxide
Zinc oxide has two crystalline forms; one is a hexagonal shaped wurtzite and one is a cubic zinc blende (see Figure 2).
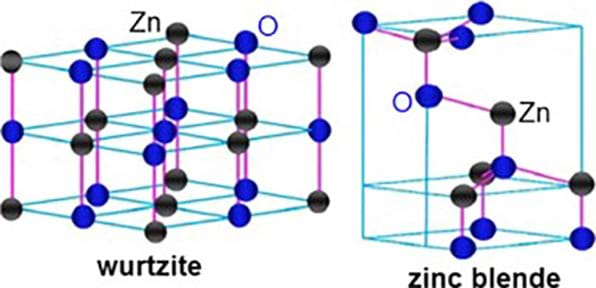
Due to its unique chemical composition, zinc oxide is a nanoparticle that exhibits a wide variety of nanostructures such as nanorods, nanowires, nanobelts and nanotetrapods. Optics, electronics, and photonics are some of the fields that zinc oxide has helped improved performance. Currently, research shows that the cytotoxic properties—any property that is toxic to living cells—of zinc oxide and its composition makes this chemical compound a candidate for being an anticancer agent and for drug delivery applications. Other nanotechnological uses for zinc oxide include antimicrobial and food preservation agents. Zinc oxide nanoparticles have recently been incorporated into textiles like cotton and wool as UV absorbers and shielding.
Titanium Dioxide
Titanium dioxide has two naturally occurring structures. Anatase and rutile structures are slightly different in their composition based on differences in their geometric configurations (see Figure 3).
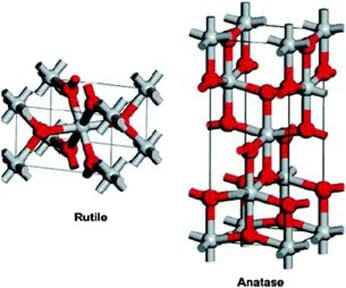
Titanium dioxide is a nanoparticle with multiple physical and chemical properties, including chemical stability, electrical conductivity, photocatalytic activity and photosensitivity. Recent applications using titanium dioxide demonstrate its effective sanitation properties. Common uses of titanium dioxide include cosmetics, sunscreens and lotions. It is also used as a pigment in different materials. Recent scientific studies reveal the useful application of titanium dioxide in the medical field as a biosensor for glucose as well as an antimicrobial agent. Titanium dioxide’s catalytic properties make it an excellent candidate in the car industry for production removing harmful toxins from exhaust emissions.
Procedure
Before the Activity
- Prepare the nanoparticle solutions for the lab activity. Note: the measurements are per group.
- Measure 5g of each nanoparticle powder and pour each into 20 ml of distilled water. You should have three separate solutions of each nanoparticle.
- Gently stir each sample solution with a pipette and label the samples.
- Prepare materials for dye solutions demonstration. (Explain the amounts and procedure to students.)
- Use a plastic pipette (approximately 3 ml) full of dye and pour into each cup with 20 ml of distilled water.
- Set materials on lab tables for student groups of four (maximum).

With the Students
- Read through the Lab Activity Handout and have students fill in the blanks as you give them instructions.
- Model what the procedure will look like once students begin the activity. Do this by having your own set of dye solutions and make one as they observe the process (see Before the Activity). Explain each step carefully so they fully understand the procedure, reproduced below.
- Clarify any questions regarding the activity. Ensure that students understand the process of mixing each nanoparticle into their newly made solutions with each dye. Remind students where and how their data will be recorded on their lab reports; suggest that they write down any observations they recorded during their experiment.
Procedure (adapted from Lab Activity Handout):
- Have students collect all materials and pour 20 ml of distilled water into each of the eight plastic cups.
- Ask students to pour 3 ml of methyl orange into four of the cups; pour 3 ml of methylene blue into the other four cups.
- Students should label the methyl orange cups as ZnO, MgO, TiO2, and “Control.” They should label the methylene blue cups the same.
- Using a pipette, tell students to place 3 drops of each sample into the cups as labelled (for example, put three zinc oxide drops into the ZnO cup; do the same with the magnesium oxide in the MgO cup and titanium dioxide in the TiO2 cup). They should stir/mix the solutions well with the used pipette.
- Have students take a picture of the solutions and controls (pre-UV light).
- Tell students to carefully take the cups outside for exposure to UV light. Using a stopwatch, measure the length of time (in minutes and seconds) it takes for each sample to bleach (do not run longer than 10 minutes).
- Have students record the time in the given data table; take another picture of the solutions and control. Have them complete their illustrations based off of their pictures.

Vocabulary/Definitions
bleaching: The process of whitening or lightening in color by exposure to sunlight (UV light) or by a chemical process.
nanocomposite: Materials that incorporate nanosized particles into a matrix of standard material; the resulting material possess in an improvement in properties such as mechanical strength, toughness and electrical or thermal conductivity.
nanoparticle: A microscopic particle whose size measures in nanometers, usually less than 100 nanometers.
nanostructure: Materials or structures that have at least one dimension between 1 and 100 nm.
photocatalytic: The ability of a substance to degrade specific compounds or organisms when exposed to UV light.
Assessment
Pre-Activity Assessment
How Do We Clean Our Water? Discussion: Ask students the following questions:
- What types of methods are used to sanitize and purify our water resources?
- How effective are these methods?
- How accessible is water throughout the world to sustain our growing population?
- What are some of the challenges a growing population faces with an increased need for clean water?
Activity Embedded Assessment
Handout: Have students work through the Lab Activity Handout. (Teacher Reference)
- Explain where all materials are located in the lab area and begin with identifying the materials needed for the experiment, safety materials, safety hazards and proper procedures for the lab protocol.
- Explain the purpose of the experiment and have students fill out the hypothesis section.
- Which nanoparticle will photosanitize water the fastest after UV light exposure: titanium dioxide, zinc oxide or manganese oxide?
- Discuss the procedures for disposal of used chemicals and clean up after they complete the activity.
- Remind students to write a conclusion discussing their results and any variables that influenced their data.
Post-Activity Assessment
Closing Questions: After the activity concludes, ask students to take five minutes and write down their individual answers to the following questions.
- What is nanotechnology?
- What is a nanoparticle?
- Which nanoparticle was the best photocatalyst?
- How can you justify your results using the background information on that specific nanoparticle and its structural composition?
- Create an explanation for your results using the structural compositions of each specific nanoparticle.
- How is this justification process used in the engineering and production of nanoparticles in our industry today?
- What are some example products of water purification and sanitation that are essential for our world today? (Potential answers: Commercial and household water filters, water treatment and purification plants, and water sanitation products essential for manufacturing companies that need to clean byproducts before releasing them into the environment.)
Poster: Review the students’ answers to gauge their comprehension of the material. Have students create a poster to express their understanding of the material, include the following: one way nanotechnology can facilitate access to clean water; write a short opinion about the future research and development of a solution to this problem.
Safety Issues
- Have students wear goggles, aprons and safety equipment while working with nanoparticles.
- Expect students to conduct themselves according to the school's safety rules/guidelines for any lab activity.
Activity Extensions
Engineering Application: Assign students to come up with their own concentration amounts for each nanoparticle sample (calculated in moles) and try to figure out whether that specific variable is indicative of the nanoparticle’s photocatalytic properties. Expect students to each write a proposed hypotheses, modify the procedure and repeat the experiment to finalize a conclusion and discussion.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!References
AZoNano. “Magnesium Oxide Nanoparticles-Properties, Applications.” AZoNano.com, 15 April 2013,
https://www.azonano.com/article.aspx?ArticleID=3353.
AZoNano. “Zinc Oxide (ZnO) Nanoparticles – Properties, Applications.” AZoNano.com, 10 July 2013,
www.azonano.com/article.aspx?ArticleID=3348.
AZoNano. “Titanium Dioxide Nanoparticles-Properties, Applications.” AZoNano.com, 9 July 2013,
https://www.azonano.com/article.aspx?ArticleID=3357
Becheri, A., Dürr, M., Lo Nostro, P., Baglioni, P. “Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV absorbers.” Journal of Nanoparticle. 2008 Springer
Weir, A., Westerhoff, P., Fabricius, L., Hristovski, K., and Von Goetz, N. “Titanium dioxide nanoparticles in food and personal care products” Environmental Science and Technology.” 2012 ACS Publications
Jin, T., He, Y. “Antibacterial activities of magnesium oxide nanoparticles against foodborne pathogens” Journal of Nanoparticle Research.” 2011 Springer
Copyright
© 2017 by Regents of the University of Colorado; original © 2016 Rice UniversityContributors
Josie ZamoraSupporting Program
Engineering Research Center for Nanotechnology Enabled Water Treatment Systems (NEWT) RET, Rice UniversityAcknowledgements
This curriculum was based upon work supported by the National Science Foundation under Rice University Engineering Research Center for Nanotechnology Enabled Water Treatment Systems (NEWT) RET grant no.1449500. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Special thanks to Dr. Juan Noveron, Luis Barrera, Carolyn Nichol and Christina Crawford.
Last modified: July 21, 2023


User Comments & Tips