Quick Look
Grade Level: 6 (5-7)
Time Required: 2 hours
(can be split into different sessions)
Expendable Cost/Group: US $1.00 The activity requires some non-expendable (reusable) materials; see the Materials List for details.
Group Size: 3
Activity Dependency: None
Subject Areas: Biology, Chemistry, Life Science, Science and Technology
NGSS Performance Expectations:

| MS-LS1-2 |

Summary
Students make edible models of algal cells as a way to tangibly understand the parts of algae that are used to make biofuels. The molecular gastronomy techniques used in this activity blend chemistry, biology and food for a memorable student experience. The models use sodium alginate, which forms a gel matrix when in contact with calcium or moderate acid, to represent the complex-carbohydrate-composed cell walls of algae. Cell walls protect the algal cell contents and can be used to make biofuels, although they are more difficult to use than the starch and oils that accumulate in algal cells. The liquid juice interior of the algal models represents the starch and oils of algae, which are easily converted into biofuels.Engineering Connection
Biological systems engineers use their knowledge of biology and the various types of chemicals and plant structures to engineer renewable energy sources like biofuels. During this activity, students use chemistry to make an edible model of an algal cell that represents the starch and oils (materials easy to convert into biofuels) and cell walls of algae (more difficult to convert into biofuels).
Learning Objectives
After this activity, students should be able to:
- Identify the parts of algae that can be made into biofuels.
- Explain that engineers need to know what the cell wall is made of in order to make biofuels easily.
- Describe how engineers—and students—use models to help them understand complex structures and problems.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-LS1-2. Develop and use a model to describe the function of a cell as a whole and ways parts of cells contribute to the function. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop and use a model to describe phenomena. Alignment agreement: | Within cells, special structures are responsible for particular functions, and the cell membrane forms the boundary that controls what enters and leaves the cell. Alignment agreement: | Complex and microscopic structures and systems can be visualized, modeled, and used to describe how their function depends on the relationships among its parts, therefore complex natural structures/systems can be analyzed to determine how they function. Alignment agreement: A system can be described in terms of its components and their interactions.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the effects of technology on the environment.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
California - Science
-
Develop and use a model to describe the function of a cell as a whole and ways parts of cells contribute to the function.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- paper napkins, one per student
- dessert-sized plates or paper towels, one for each student, on which to place and eat the algal models
To share with the entire class:
- food grade sodium alginate (need 5 g for 1 liter of juice); such as 2 oz (56 g) for $12 at Amazon
- food grade calcium lactate (need 20 g for 2 liters of water); such as 1.8 oz (51 g) for $10 at Amazon
- low-acid fruit juice (pH > 3.6), such as pear, apple or carrot), ~100-250 ml juice per student; ~1 gallon is plenty for a 30-student class; for a no-sugar option, use distilled water that is safe for drinking (sodium or potassium added); alternatively, use tap water, although high calcium (hard water) can cause premature solidifying
- (optional) liquid food coloring, if using water instead of juice and want to color it
- water, ~4 liters
- 3 large bowls, each ~1-4 liters in size
- whisk or blender
- 1-2 three-inch fine mesh tea strainers or small sieves, such as the 3-inch fine stainless steel mesh strainer for $1.39 each at the Webstaurant Store
- 2 small ladles or spoons, each about 1-2 fl. oz in size
- laboratory scale, accurate to 1 g; alternatively, use a kitchen scale such as the Jennings CJ4000 4000g x 0.5g digital scale for $26 at Amazon
- paper or cloth towels, for clean-up
Cost note: The main expendable costs are for juice, paper towels and small amounts of the two reagents. The initial cost of the reagents is high, but only a very small portion is used.
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/ucd-1811-algae-edible-model-cell-biofuel] to print or download.Pre-Req Knowledge
Students need to know the background information about algae, biological engineering and biofuels, as presented in the associated lesson, Algae: Tiny Plants with Big Energy Potential.
Introduction/Motivation
We’ve learned (in the associated lesson) that algae are tiny plants that require a microscope to see their cellular components. Today, we are going to make edible models of algae so that we can better understand the cell structure of algae.
Why do people make models? Engineers create models for many reasons: to be able to simplify complex problems, to help them visualize and experiment with things that are too big or too small to make in their real-world sizes, and to test different ideas in controlled environments. Some engineers program computer models, others build physical models, but we have it the best today because we are going to create models that you can eat!
First, let’s learn some background information and recall what we learned about algae.
(Slide 1) Today we are going to make edible models of algae like the students you see in this photograph. The other photo is a close-up view of the edible algal models, which are made with water and food coloring, but we are going to use ___ juice (fill in the blank with the juice type purchased).
(Slide 2) Let’s review what we remember/know about algae and biofuels. What are the names of the three parts of algae that can be made into fuel? Here’s a cartoon drawing of a plant cell. (click to advance) If we ignore the cell organelles, we see this simplified view of a plant cell. The parts of the algae that we can use to make into fuel are (click to advance) oil, (click) starch and (click) cell walls. Also, remember that we need to break apart the cell walls to get to the oils and starch, plus we can use the broken-down cell walls for fuel, too. (Teacher note: The depicted plant cell is shown with adjacent cells; algae can be single-celled or multicellular.)
(Slide 3) Who remembers the definition for a cell wall? Talk with your neighbor about the definition of the cell wall. (Wait a few minutes.) The definition of the cell wall is (click to reveal answer) a structure made of sugar that surrounds and protects the cell, and (click) its sugars can be used to make more biofuel. Biological engineers study algal cell walls to find ways to more easily make biofuel by opening the cell wall to get to the oil and starch, and by recycling the sugars of the cell wall into biofuel.
(Slide 4) Today, we are going to create models of algal cells. We will be able to touch and feel the cell walls in our models.
See how the cell wall of the algal model protects the cell’s interior? That protection is important for the cells to live. Because the cell wall is such a tough material to break down, biological engineers spend lots of time researching it.
(Slide 5) What are polymers? Here are a few clues. (click to advance) First, this is a monomer. What does the prefix mono mean? (Answer: One.) (click) Next, we have a dimer. What does the drawing show? (Answer: The prefix “di” means two.) (click) Finally, we have a polymer. Discuss with your group or neighbor what you think polymer means.
(Slide 6) Here is a scientific definition for a polymer. (click to reveal answer) A polymer is a large chemical made of many repeating parts. Just like we reviewed in the last slide, the prefix “poly” means many and “mers” are small, repeating pieces. (click to advance) Some examples include the cell wall, DNA, protein and LEGO® pieces. Can you think of any other examples? (Possible answers: Beads on a necklace, plastic molecules, etc.)
(Slide 7) Here is some background information on the materials we are using today to make edible cell models. (click to advance). The polymer we are using today is called sodium alginate. It is made from algae (coincidentally) and is completely edible. We can make a gel using the sodium alginate by exposing it to calcium lactate (also edible).
(At this point, administer the pre-activity quiz, as described in the Assessment section.)
Procedure
Background
This activity uses a molecular gastronomy technique called spherification to form edible models of algal cells. The final products are a bite-sized, juice-filled spheres that can be eaten. The gelling agent used to make the algal models out of juice is called sodium alginate, which is an edible powder made from brown seaweed. Mixed with water or low-acid juice, it is a non-viscous liquid, but when it is exposed to calcium, it forms a gel in seconds. This process is useful for classroom instruction to create edible cell models in which the gelled sodium alginate represents the algal cell wall and the liquid interior represents the lipids and starch inside algal cells. The analogy goes further because like algal cell walls, the model walls protect the interior of the cell and are hard (denser) to break down into chemicals for biofuels.

- Gather materials and make copies of the handouts, one each per student: Before You Begin Pre-Quiz, Activity Worksheet, Activity Post-Quiz.
- Set up one algae model-making station on a long counter or table through which you will cycle students in groups of 2-5 (see Figure 1). See detailed preparation steps below.
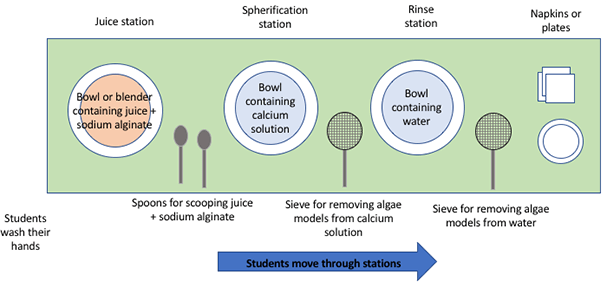
Prepare the Station
Ideally, 1) locate the station near a sink for setup/clean-up convenience and so students can first wash their hands before beginning, and 2) make the station accessible from both sides so the instructor can help students through the process without getting in the way. Refer to Figure 1.
- Wipe the counter clean and lay down clean towels to minimize any mess.
- At the end of the station, put out a supply of napkins and/or plates for serving the juice spheres.
- Set out spoons and sieves for scooping the juice + sodium alginate mixture into the setting solutions and for removing the algal models from the calcium solution and the rinse water.
- Fill a bowl with water for the rinsing sub-station. If desired, provide multiple rinse stations. The calcium lactate tastes slightly bitter so well-rinsed models taste better.
- Make a ~1% solution of calcium lactate by combining 10 grams of calcium lactate and 1 liter of water. This is the “calcium solution.”
- Immediately prior to students beginning the activity, make a ~0.5% sodium alginate-juice solution by combining 5 grams of sodium alginate with 1 liter of juice. Whisk or stir well to combine. If you have a large class, it helps to have several batches of sodium alginate already measured and ready to be added to already-measured liters of juice.
- Test the spherification process by scooping 1-2 tablespoons of juice into the bowl containing the calcium solution. Wait a few seconds and remove and rinse the juice ball. If the juice does not form a ball, add 1 gram increments of sodium alginate until juice spheres form easily.
With the Students
- Present to the class slides 1-7 of the PowerPoint® presentation.
- Then administer the pre-activity quiz.
- (Slide 8) Review with the class the activity steps. Tell the students: This slide lists the steps for making the algae juice sphere models. Let’s go over the steps. (click to advance)
Step 1: Wash your hands.
Step 2: Put a scoop of the juice + sodium alginate mixture into the calcium solution.
Step 3: Wait about 30 seconds. Then use a strainer to remove the juice sphere from the calcium solution. Put it into the water to rinse.
Step 4: Use a strainer to remove the juice sphere from the water. Make observations about your algal model and then eat it!
- Organize the class into groups of 2-5 students each. Hand out the activity worksheets.
- Group by group, send students through the algae model-making station. Guide students through the specific steps:
- Students begin by washing their hands.
- Students take a scoop, ~1-2 tablespoons, of sodium alginate and put it into the bowl containing the calcium solution.
- After waiting ~30 seconds, students use the sieve to place the juice spheres into the bowl containing water.
- After rinsing, students use a sieve to put the juice spheres on to small plates or napkins.
- After a group has made its algal models, have them step away from the station with their models, and move to their desks or another seating area. Direct them to eat the spheres immediately and record their observations as prompted on the worksheet. Have them answer all worksheet questions.
- (Slide 9) After all groups have finished the algae model-making activity and completed the worksheet, watch the nine- and six-second videos on this slide. Facilitate a class discussion, as described in the Assessment section.
- Conclude by administering the post-activity quiz, as described in the Assessment section.
Vocabulary/Definitions
cell wall: A structure made of sugar that surrounds and protects a cell. Cell wall sugars can be used to make biofuel.
polymer: A large chemical made of repeating pieces. Examples include materials in the cell wall, DNA, protein and LEGO pieces.
spherification: A molecular gastronomy technique that shapes liquids into spheres usually using sodium alginate and either calcium chloride or calcium glucate lactate, which visually ad texturally resemble roe (fish eggs).
Assessment
Pre-Activity Assessment
Pre-Quiz: After showing students slides 1-7 of the Edible Algae Models Presentation, administer the Before You Begin Pre-Quiz. The quiz asks students to each demonstrate what they recall/know about algae and biofuels from the associated lesson and the presentation, and to draw a picture of an algal cell with labeled parts (oil, starch, cell wall-sugar). Review their answers to gauge their comprehension.
Activity Embedded Assessment
Worksheet: After making and eating their algal cell models, have students complete the Activity Worksheet to recall the activity steps (method), their observations about the spherification process, four-senses observations about the model, and an updated drawing of the algal cell model to identify what each part represents in the real algal cell. Review their answers to assess their comprehension.
Post-Activity Assessment
Videos & Discussion: After the activity, show the class the nine-second Juice Sphere Video 1 and the six-second Juice Sphere Video 2 (both on slide 9). Then facilitate a class discussion about how the juice spheres model the parts of algae that biological engineers are inventing ways to use for biofuels. Consider going over the questions/answers on the Activity Worksheet Answer Key as a class.
Post-Quiz: Administer the Activity Post-Quiz, which asks students about the uses for models, how this model impacted their understanding of the algal cell, and what could be done to improve the model. Alternatively, use these questions/answers to further the class discussion.
Safety Issues
- Even though all the chemical compounds used in this activity are edible and safe to handle, it is a good practice to prepare the solutions in advance for student use.
- Conduct this activity in a location that is safe for eating, for example, not in a laboratory that contains hazardous chemicals.
- Consider students’ food allergies when selecting the juice type.
Troubleshooting Tips
If the juice spheres do not solidify, add 1gram increments of sodium alginate to the juice, stirring and testing after each addition.
If the juice solidifies too rapidly after addition of sodium alginate, the juice may be too acidic. Options include using distilled water, lower-acidity juice, or diluting the acidic juice with distilled water. Alternatively, food grade sodium citrate may be added to the juice to increase the pH. It may be helpful to have pH paper handy to determine the juice pH; for spherification, the optimum juice pH is greater than 3.6.
Activity Scaling
- For lower grades, reduce or decrease the complexity of the terms presented. Make available additional classroom aides to help younger students create cell models.
- For higher grades, have students research polymers online and report to the class other common polymers and their uses. Possible examples for research include DNA, PET polyester, cellulose in paper or cotton, and pectin in jam.
Additional Multimedia Support
The 26-second Algae Activity Video shows a series of scenes: the associated lesson being presented in a fifth-grade classroom and students conducting this activity—making algal cell models and eating them!
For additional spherification tips, consult http://www.molecularrecipes.com/spherification-class/basic-spherification/ and watch an informational video. Note that some recipes on the web page and video contain alcohol. On the other hand, spherified cocktails with fellow teachers can be a fun addition to staff get-togethers!
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to biofuels, biological engineers, algae and how they grow (photosynthesis), and what parts of algae can be used for biofuel (biomass from oils, starches, cell wall sugars). Through this lesson, plants—and specifically algae—are presented as an energy solution. This lesson pr...

Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics.
Copyright
© 2017 by Regents of the University of Colorado; original © 2016 University of California DavisContributors
Lauren JabuschSupporting Program
RESOURCE GK-12 Program, College of Engineering, University of California DavisAcknowledgements
The contents of this digital library curriculum were developed by the Renewable Energy Systems Opportunity for Unified Research Collaboration and Education (RESOURCE) project in the College of Engineering under National Science Foundation GK-12 grant no. DGE 0948021. However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Thanks to Cristian Heredia for recording and editing the short video.
Last modified: February 18, 2021




User Comments & Tips