Quick Look
Grade Level: 11 (10-12)
Time Required: 2 hours 30 minutes
three 50-minute class periods
Expendable Cost/Group: US $1.00
Group Size: 3
Activity Dependency: None
Subject Areas: Chemistry, Computer Science, Physical Science, Science and Technology
NGSS Performance Expectations:

| HS-PS1-6 |
Summary
Students use an online molecule-modeling platform to learn about alkanes. Students then model the reaction of ethylene into butylene through a chemical process called oligomerization, which is one step in the process of converting shale gas ethane into fuel for transportation.Engineering Connection
The transportation industry gets 85% of its fuel from fossil resources. Shale gases, found readily in the United States, are an emerging resource projected to provide enough energy to bridge the gap from our reliance on fossil fuels to reliance on renewable resources for transportation. Shale gases are composed of light hydrocarbons which cannot replace octane directly. Chemical engineers design methods to process light shale gases into octane-level fuels. These engineers develop new catalysts and reactor designs with the help of scientists who iterate computer simulations to maximize conversion rates and reduce the fuel needs of the process itself. With their innovations and hard work, we will reach our goal of environmental sustainability.
Learning Objectives
After this activity, students should be able to
- Model molecules using the WebMO website.
- Describe how light hydrocarbons are converted into fuels for transportation.
- Recognize alkanes as compounds composed of carbon and hydrogen.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
CCC.7.9-12.6.
Much of science deals with constructing explanations of how things change and how they remain stable.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
DCI.PS1.B.9-12.1.
Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-6. Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: In many situations, a dynamic and condition-dependent balance between a reaction and the reverse reaction determines the numbers of all types of molecules present.Alignment agreement: Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed.Alignment agreement: | Much of science deals with constructing explanations of how things change and how they remain stable. Alignment agreement: |
State Standards
Indiana - Science
-
Describe, classify and give examples of various kids of reactions-synthesis (i.e., combination), decomposition, single displacement, double displacement and combustion.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Predict products of simple reactions such as synthesis, decomposition, single replacement and double replacement.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Balance chemical equations using the law of conservation of mass and use them to describe chemical reactions.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- laptop or computer
- web browser, Google Slides
- 50 gumdrops, two colors
- 50 toothpicks (or 25 toothpicks split in two)
- digital camera or phone with camera to take picture of gumdrop molecule
For the class to share:
- laptop or computer
- projector (to show CISTAR and WebMO instruction videos)
- access to the WebMO site: https://www.webmo.net/demoserver/cgi-bin/webmo/login.cgi (see the Procedure for more information)
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/und-2664-alkane-resources-molecules-activity] to print or download.Pre-Req Knowledge
A basic understanding that matter is composed of atoms which bond together to form compounds, which can react and form different compounds.
Introduction/Motivation
How often do you need to ride in a car or a bus to get where you are going? For many people the answer is a lot: especially in suburban and rural areas and places with underdeveloped public transportation infrastructure. People need cars to get to work, to the grocery store, to the doctor, and to nearly everywhere else. But gasoline comes from crude oil, which is expensive, eventually be completely depleted, and is a big contributor toward greenhouse gas emissions. What can we do to stop using so much crude oil?
Do you ever wonder if we will ever resolve these environmental problems? It is scary to think what may happen if we do not. Did you know we are currently relying almost entirely on fossil fuels for transportation? Even though we know those fossil fuels will not last forever. What are some resources that are renewable? (Possible answers: solar power, wind energy, biofuels.) Those resources sound promising, but we do not yet have the technology to use those resources for transportation.
Have you ever heard of shale? Or fracking? (Let students offer answers. Responses will vary.) Shale is a type of rock found underground that is infused with oil that is very similar to petroleum oil. A significant part of that shale oil is known as shale gas. Shale gas contains methane, ethane, propane, and butane. Initially, only the methane part was thought to be useful. (The methane is widely used as natural gas to heat our homes.) The other compounds were treated as waste products! Plus, the other compounds were hard to transport because ethane and propane are gases at room temperature, and they cannot be used as gas in a car, or in the furnaces that heat most buildings.
For decades, we have relied on petroleum that mostly comes from outside of the United States for transportation; however, engineers have now developed the technology to convert those unwanted shale gases into gasoline. We have enough shale gas in the United States to last about 100 years as fuel for transportation. Since current projections estimate we won’t rely on renewable resources, like solar and wind, until about 2060, these shale resources will help us bridge the gap from our reliance on foreign fossil fuels to renewable resources.
Today we will start to learn more about how fuels can be made from shale gas. By the time we are done with this activity, you will be able to tell your friends all about the reactions that transform ethane into butylene, which is the first step in transforming shale gas into octane for our automobiles.
Procedure
Background
Shale is rock formation found underground that is infused with shale oil. Part of that shale oil is known as shale gas, and it contains methane, ethane, propane, and butane. The ethane in shale found in the United States is abundant enough to supply our country with fuel for transportation for about 100 years, but it must be converted into fuel for that to happen. This activity will demonstrate the conversion of ethane into butylene.
Before the Activity
- Watch the attached video WebMO Instructions (4:53) to become familiar with WebMO.
- Gather materials and make copies of the Alkane Resources Pre-Quiz and the Project Notebook.
- Prepare sets of gumdrops (50 each black and white) and toothpicks (around 50, or 25 split in two) for each group.
With the Students
Day 1
- Pass out the Alkane Resources Pre-Quiz to each student.
- Give students 15 minutes to complete the pre-quiz and turn it in.
- Divide the class into groups of three each.
- Show the CISTAR Introduction video: https://www.youtube.com/watch?v=lEZEPE9rdR0
- Discuss the outlook of converting to renewable resources.
- Share the Introduction Slides with the students, which are a summarized version of the video. Leave the last slide of the vocabulary definitions on the projector for students to see.
- Hand out the Project Notebook to each group.
- Have groups answer the questions for Day 1 in the Project Notebook using the video and presentation information.
Day 2
- Assign each group an alkane compound from this list:
- propane
- 2-methyl propane
- butane
- 2-methyl butane
- pentane
- 2-methyl pentane
- hexane
- 2-methyl hexane
- heptane
- octane
- Have students answer the questions in the first section of Day 2 in the Project Notebook.
- Show the students how to use WebMO or have them watch the attached video WebMO Instructions (4:53).
- Give students time to construct their molecules in WebMO. Instructions are in the Project Notebook or below:
- Go to WebMO Demo Server: https://www.webmo.net/demoserver/cgi-bin/webmo/login.cgi
- Log in with these credentials:
- Username: guest
- Password: guest
- Type <enter>
- Across the top should read “New Job”, “Refresh”, “Download”, etc.
- Select “New Job”.
- Click “Create New Job”. This will go to the “Build Molecule” page.
- Click the blank screen once for each carbon atom in the molecule.
- Move the cursor between clicks so the carbon atoms are in a line.

- Draw a chemical bond between each of the atoms: Click and hold the cursor on the first atom and drag the cursor to the next atom. Control-Z will reverse any mistakes.

- Select “Build”, then select H for hydrogens.
- Click the blank screen once for each hydrogen atom, spreading them evenly around the carbon atoms.
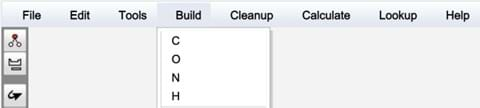
- Draw a chemical bond between each hydrogen and the nearest carbon atom.
- Under “Cleanup”, select “Geometry” to correct the shape of your molecule.

- Under “Lookup”, select “Molecule Info” and record the following information about your molecule:
- Stoichiometry:
- IUPAC Name:
- Molar Mass:
Day 3
- Ask students to answer the first two questions of the Day 3 portion of the Project Notebook.
- Pass out one set of 50 gumdrops and 50 toothpicks to each group.
- Allow each group to decide which colors represent carbon and hydrogen atoms.
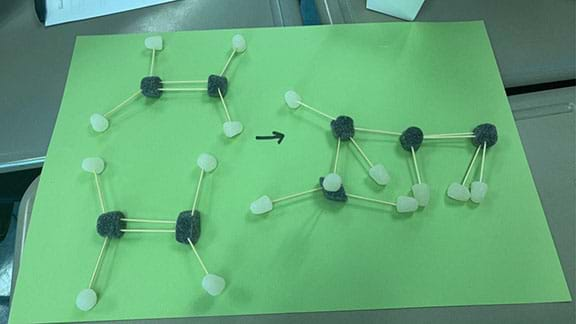
- Have students fill in the table in step three of the Day 3 portion of the Project Notebook with the number of carbon and hydrogen atoms for both ethylene and butylene.
- Use the gumdrops and toothpicks to model ethylene and butylene molecules.
- With their leftover gumdrops, have students design models how ethane can be converted into octane.
- Have students set up their molecule models with an arrow from the starting products to the ending products.
- Have them check their reaction model using the Gumdrop Reaction Rubric in the Project Notebook.
- Have students correct their reaction model until it has a score of at least 8 points.
- Have each team captain check their reaction model with the teacher.
- Have students take a photo of their model reaction.
Vocabulary/Definitions
alkane: Any acyclic saturated hydrocarbon (e.g. methane, ethane, propane, butane, etc.)
chemical reaction: A process, typically involving the breaking or making of interatomic bonds, in which one or more substances are changed into others.
compound: Any substance formed by the union of two or more chemical elements in a fixed ratio, the union being a chemical bond.
element: Any one of the simplest chemical substances that cannot be decomposed in a chemical reaction. Chemical elements consist of atoms which have the same number of protons.
monomer: A relatively small molecule which can be covalently bonded to other monomers to form a polymer.
non-renewable resource: A resource that will not return, or renew, or will only return after a long period of time.
oligomerization: The formation of an oligomer from a monomer.
renewable resource: A natural resource that is replenished by natural processes at a rate comparable to its rate of consumption by humans or other users.
Assessment
Pre-Activity Assessment
Pre-Quiz: Have students take the Alkane Resources Pre-Quiz to gauge their understanding.
Activity Embedded (Formative) Assessment
Notebook: The formative assessment is embedded in Day 1 of the Project Notebook. It includes these questions:
- Evaluate your own current understanding of the problem:
- Light shale gases like ethane need to be converted into fuels and petrochemicals. How can that be done?
- What do you know about this problem?
- What do you need to know about this problem?
Project Vocabulary: Students discuss with their team the main differences, if any, between the meanings for these words that you wrote on your pre-assessment and their actual definition provided in the Project Glossary (last slide in the Introduction Slides). Then score each word from 1-3 for level of understanding.
Post-Activity (Summative) Assessment
Rubric: The Post-Activity Assessment includes a Project Rubric for students to self-assess their gumdrop model of the balanced reaction and a photo of the model for the teacher to evaluate. The teacher should evaluate the reaction model using the rubric the students use to self-assess their reaction.
Investigating Questions
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn and discuss the advantages and disadvantages of renewable and non-renewable energy sources. They also learn about our nation's electric power grid and what it means for a residential home to be "off the grid."

Students are introduced to the concepts of air pollution, air quality, and climate change. The three lesson parts (including the associated activities) focus on the prerequisites for understanding air pollution. First, students use M&M® candies to create pie graphs that express their understanding o...
Copyright
© 2023 by Regents of the University of Colorado; original © 2021 University of Notre DameContributors
Rebecca HumbargerSupporting Program
Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR), University of Texas at Austin, Purdue University and the University of Notre DameAcknowledgements
This activity was developed as part of a 2021 Research Experience for Teachers (RET) carried out by the Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR). The Principal Investigator was William Schneider, Ph.D., the faculty mentor was Hanyu Ma, and grad student mentors were Neha Mehra and Craig Waitt from the University of Notre Dame.
Last modified: March 15, 2023




User Comments & Tips