Quick Look
Grade Level: 10 (9-10)
Time Required: 1 hours 30 minutes
Expendable Cost/Group: US $0.00
Group Size: 2
Activity Dependency: None
Subject Areas: Chemistry, Measurement

Summary
Students use a hot plate, ice-water bath, and common lab equipment to prepare a viscous or semi-viscous liquid at cold, room, and warm temperature levels. Students measure the mass, volume, and density of their specific liquid to determine the viscosity of their liquid at different temperatures. Students compare their liquid to another group’s liquid to compare and determine any trends with temperature and viscosity.Engineering Connection
Students gain foundational knowledge that can be applied to real-world engineering challenges, emphasizing the interdisciplinary nature of engineering and its reliance on fundamental scientific principles like fluid dynamics. Engineers must have a strong understanding of viscosity in various temperature conditions, especially in industries where fluid dynamics play a crucial role. Engineers designing systems involving liquids, such as pipelines, chemical processing plants, or even food manufacturing, need to consider how temperature variations affect viscosity.
Learning Objectives
After this activity, students should be able to:
- Describe the factors that affect viscosity.
- Describe the relationship between the temperature of a fluid and its viscosity.
- Make basic measurements measuring mass, volume, time and temperature.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
DCI.PS1.B.9-12.1.
Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
SEP.1.9-12.1.
Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
SEP.12.9-12.4.
Use mathematical representations of phenomena or design solutions to describe and/or support claims and/or explanations.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
express and manipulate chemical quantities using scientific conventions and mathematical procedures, including dimensional analysis, scientific notation, and significant figures;
(Grades
10 -
12)
More Details
Do you agree with this alignment?
-
understand energy and its forms, including kinetic, potential, chemical, and thermal energies;
(Grades
10 -
12)
More Details
Do you agree with this alignment?
-
perform calculations involving heat, mass, temperature change, and specific heat; and
(Grades
10 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- a scale (electronic or triple-beam)
- a weighing tray
- a 100 ml graduated cylinder
- a steel or marble ball (about 1-inch diameter)
- a 500 ml or 600 ml beaker
- 1-3 150 ml beakers
- a ruler
- an electronic or glass thermometer
- a hot plate
- a dry-erase marker
- a stopwatch app or device
- a pen or pencil
- one Let it Flow! Worksheet per student
- safety equipment:
- a pair of safety goggles per student
- a lab safety apron per student
- a pair of tongs or a heat mitt per group
For the entire class to share:
- 1-2 large plastic container ice/water bath
- 2-4 viscous or semi-viscous liquids (i.e., honey, oil, corn syrup, clear dish soap, etc.) at least a gallon each.
- optional: a bottle of ketchup and a bottle of salad dressing
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/uoh-2795-fluid-viscosity-temperature-activity] to print or download.Pre-Req Knowledge
Lab safety
Use of equipment such as scale, thermometer, and hot plate
Basic math skills
General knowledge of fluid properties
Introduction/Motivation
(If possible, have a bottle of ketchup and salad dressing as props to show students.)
Suppose you were a chemical engineer working at a company called Chemical X. Your company produces a sandwich spread named ‘Gloopy-Glop’ that is used on sandwiches for lunch in high school. Your company is attempting to pump it through its piping system to fill jars for shipping.
However, the spread is very thick, and it is not pumping through the pipes very well. What could be done to fix this problem? (Let students offer answers.)
What pours out faster: ketchup from a bottle, or salad dressing? Why? (Note: Students may not understand or know the answer to the first question. That’s okay. This question is just to see what they know deductively about viscosity or how they would go about problem solving. However, whether they get it wrong or right, do not give the answer away! Tell them you will get back to the answer soon. As far as the second question, students may say that ketchup pours more slowly because it is “more dense” or ”thicker” than salad. )
Write down your answers to these two questions. (Give students five minutes to write their answers to the two questions on a sticky note, note card, or piece of paper.)
Now we are going to get into groups of two to four, and I want you to share your answers with each other in a round-robin style. Once you have shared your answers, pick one person in your group to be a representative to give your group’s answers in front of the class.
(Write the following words on the board: temperature, viscosity, mass, volume, density.)
Which property do you think is most responsible for the “thickness” or slower pouring rate of the ketchup?
(Answer: viscosity.)
The viscosity of a liquid is its resistance to flow. Various liquids have different viscosities. For example, rubbing alcohol seems to pour very easily from its container, whereas liquids such as lotion or hand sanitizer seem to flow out more slowly. Liquids with higher viscosities tend to pour more slowly, due to strong molecular bonding. The higher the viscosity of a fluid, the harder you must push it to move it. We call a higher viscosity fluid more viscous.
(Show the following video: https://www.youtube.com/watch?v=9NYs3Y-IjGw. Stop at the 1:08 mark where the video says that… “viscosity varies with temperature”)
Now, I’d like one person from each group to explain your group’s consensus answer for the second question. (Let students share their answers.)
How do you think temperature may affect the viscosity of a liquid? (Note: Students may or may not have some understanding that higher temperature means the flow pours faster.)
Today we are going to learn about viscosity!
Procedure
Background
The viscosity of a liquid is its resistance to flow. Various liquids have different viscosities. For example, rubbing alcohol seems to pour very easily from its container, whereas liquids like lotion or hand sanitizer seem to flow out more slowly. Liquids with higher viscosities tend to pour more slowly, due to strong molecular bonding. The higher the viscosity a fluid has, the harder you must push it to move it. We call a higher viscosity fluid more viscous.
There are several formulas or equations that can be used to calculate viscosity, but the most common is this equation derived from Stoke’s Law: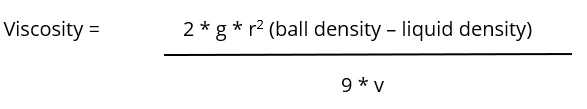
where g = acceleration due to gravity = 9.81 m/s2 (981cm/s2), r = radius of ball bearing, and v = velocity of ball moving through a liquid. The SI unit of viscosity, represented by µ, is equivalent to Newton-second per square meter (N·s m2). It is sometimes referred to as the “poiseuille” (Pl). One poise is exactly 0.1 Pa·s. One poiseuille is 10 poise or 1,000 cP, which is considered a centipose. To give perspective, water—a liquid common to us all—has a cP of 1.0, which is considered a very low viscosity. However, castor oil and somewhat “runny” honey have much higher viscosity values of 1,000 cPs and 3,000 cPs, respectively. In fact, peanut butter, which almost seems like a solid rather than a fluid, has a viscosity of 250,000 cPs!
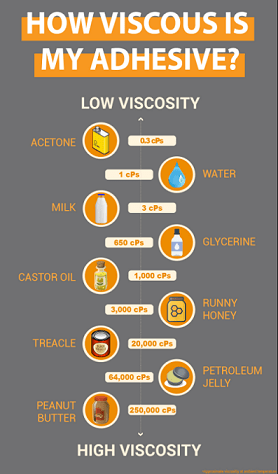
Viscosity is important because chemical and process engineers have to deal with different fluids bearing different coefficients of viscosity for applications such as road construction, roofing, waterproofing, refining oil and various fluid chemical products, and even food processing—who wants their peanut butter to flow out like water?
The viscosity of a fluid is influenced by several factors, such as a substance’s inner molecular structure, shear stress (i.e., mixing the chemical), pressure, and temperature. Although all of these are important variables for determining a liquid’s viscosity, the scope of this lab is to simply focus on the relationship between temperature and viscosity.
Because the lab procedures contain several parts, students will measure viscosity in g/cm*s (Poises) in order to directly input their measurement units into the Stokes equation and get a general understanding of viscosity based on their compared values at different temperatures and their observations.
In most cases, as the temperature of a fluid increases, its viscosity decreases. The increased thermal energy increases the kinetic energy (motion) of the molecules, giving them the ability to overcome and break the molecular bonds that hold them together. This allows these molecules to move or pour more easily. Understanding this scientific principle allows engineers to counteract this process when it would yield unfavorable results. For example, in cars, motor oil is used to lubricate the car’s engine so that it won’t overheat. However, in really hot conditions, with viscosity decreasing, regular oil will become too thin to be effective to cool it down, which could cause permanent damage to the engine. And at very low temperatures, regular oil would become too viscous to circulate evenly around the engine parts, which also creates a problem. So to deal with this issue, specially formulated motor oils have been engineered in order to actually increase viscosity when heated, and decrease when cooled. This allows our vehicles’ engines to run optimally over a wide range of temperatures.
Before the Activity
- Gather lab materials and make copies of the Let it Flow! Worksheet.
- Pour out “stock solution” amounts for each group into a predetermined container. (Note: You probably do not want students obtaining their liquid from the main supply.)
- Each group needs a 500 ml or 600 ml beaker filled with 500 ml of a viscous or semi-viscous liquid.
- Set out lab equipment (see list) in designated lab groupings. Make sure that hot plates are plugged in and working (but not turned on) and that one or two ice-water baths are set up in a designated area for the groups to use.
- Think about how you will assign groups (numbering systems, student choice), and how you will choose to have a representative answer beforehand.
- Make sure to have viscosity video(s) ready to play and be prepared to write terms on the board.
With the Students
- Give the students a general overview of the lab (you may want to point out/show the students equipment needed for each part of the activity).
- Review safety procedures and lab roles (i.e., group manager, safety director, technical writer for each group).
- Make sure that students are in their specified groups.
- During the lab, make sure you go around to monitor student progress and safety (especially with handling the hot plates), and to assist with any procedures students may have questions about. Students should follow instructions from the Let it Flow! Worksheet.
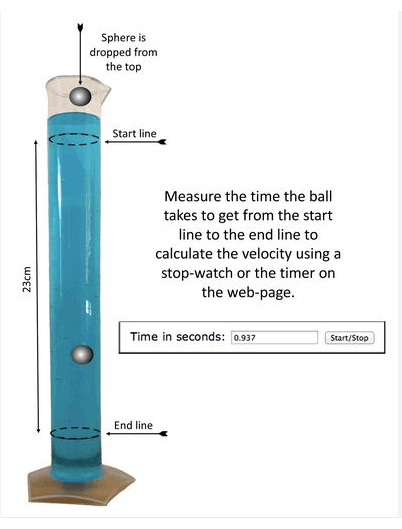
Figure 1. Illustration of how to calculate the velocity of speed of sphere to further determine a liquid’s viscosity. 
Figure 2. Illustration of liquid-filled beaker in ice-water bath. 
Figure 3. Illustration of a beaker on top of a laboratory hot plate. - Have students fill in the information with their data and calculations from the worksheet, and then answer the conclusion questions.
- Have students clean up their lab spaces as they finish their experimentation and work on the lab conclusion questions.
- After a designated time for lab completion and cleanup, ask students to give their explanation of conclusion question #3 (this question is actually the first engage question from the Introduction/Motivation section; you want to check whether students now understand how to approach the question with an answer based on the lab).
- Briefly explain to the students why higher temperature decreases the viscosity of a liquid. You can use any of the following resources:
- Honey Viscosity at Different Temperatures
- At 1:00 minute, the video starts to show the viscosities of honey at different temperatures. An explanation starts at 1:38 minutes. You may want to pause the video before the explanation to allow students to discuss in their groups first. Have students relate the decrease of the force of attraction to the greater ability of the molecules to break bonds due to increased kinetic (heat) energy.
- phET States of Matter: Phase Changes
- In this simulation, select the “Phase Change” tab at the bottom. Select the “liquid” icon. Show students how slightly adding heat causes the atoms/molecules to spread out or “break-away” from each other, which increases their ability to slide past each other if poured = lower viscosity (decreasing temperature would have the reverse effect).
- Display Figure 5, “Viscosity & Temperature,” and explain to students that higher temperature increases kinetic energy (= thermal or heat energy = thermal motion) of the molecules, allowing them to overcome molecular bonding.
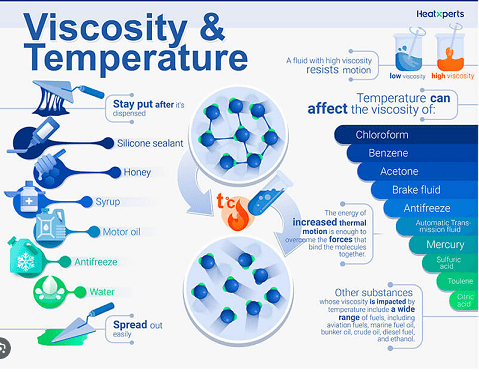
Figure 5. Visual illustration showing the effect of temperature on viscosity.
- Afterward, have students answer the Exit Ticket questions to wrap up as a final check for understanding.
Vocabulary/Definitions
density: A substance's mass per unit of volume. The symbol most often used for density is ρ, although the Latin letter D can also be used.
fluid: A substance that has no fixed shape and yields easily to external pressure; a gas or (especially) a liquid.
kinetic energy: Energy that a body possesses by virtue of being in motion.
mass: Traditionally believed to be related to the quantity of matter in a physical body.
molecular bond: A type of chemical bond involving the sharing of electrons between atoms in a molecule.
molecule: A group of two or more atoms held together by attractive forces known as chemical bonds.
temperature: Relates to the kinetic energy of the molecules.
thermal energy: The energy contained within a system that is responsible for its temperature. Heat is the flow of thermal energy.
velocity: The speed and the direction of motion of an object.
viscosity: The measure of a fluid's resistance to flowing.
volume: The amount of space that a substance or object occupies.
Assessment
Pre-Activity Assessment
Warm-Up/Engage Questions: Ask the engage questions from the Introduction/Motivation:
“Suppose you were a chemical engineer working at the company Chemical X. Your company produces a sandwich spread named ‘Gloopy-Glop’ that is used on sandwiches for lunch in high school. Your company is attempting to pump it through its piping system to fill jars for shipping.
However, the spread is very thick, and it is not pumping through the pipes very well. What could be done to fix this problem?”
“What pours out faster? Ketchup from a bottle or salad dressing? Why?”
Activity Embedded (Formative) Assessment
Hypothesis: Students will give their thoughts on their results of the lab, centered on the following questions: Do you think the temperature of a fluid has a significant effect on its ability to flow? Why or why not?
Lab Questions: Students will answer these questions on the Let it Flow! Worksheet.
Post-Activity (Summative) Assessment
Exit Ticket: Have students to complete the Exit Ticket.
Safety Issues
Please be sure to monitor the students as they use the hot plates. Students need to use beaker tongs or heat mitts when handling glassware placed on the hot plate. Also, make sure the setting is placed on “medium” instead of “high” so the liquid does not get too hot to handle.
Also, make sure students are careful with glassware to avoid breakage and injuries.
Troubleshooting Tips
Pacing
To keep students on track, set a timer for specific time segments to help students progress through the lab. Here is a suggested time breakdown (based on the Parts of the Let it Flow! Worksheet)*:
- 5 mins - Class start/warm-up
- 10 mins - Introduction
- 10 mins - Part 1
- 10 mins - Part 2
- 10 mins - Part 3
- 10 mins - Part 4
- 10 mins - Part 5
- 10 mins - Part 6
- 10 mins - Wrap-up
- 5 mins - Exit Ticket
= 90 mins Total
*Students can progress further if they finish a segment before the suggested time, but please monitor groups so they don’t fall behind.
Activity Extensions
Have the students record the viscosities and corresponding liquid temperatures for participating groups. The class can then graph these as data points, with temperature being the independent variable and viscosity being the dependent variable, in order to further analyze the class’s overall data trend.
Activity Scaling
Modifications for younger students/lower grade: Eliminate the calculation needed to find viscosity. Just heat, cool, or measure the liquid’s temperature at room temperature and record. Then pour the liquid into the graduated cylinder up to the 100 ml and time. The time of pouring will act as an indication of the fluid’s viscosity.
Additional Multimedia Support
https://efficientengineer.com/viscosity/
https://wiki.anton-paar.com/en/basic-of-viscometry/
https://www.intertronics.co.uk/2021/06/adhere-academy-what-is-viscosity/
https://www.plantengineering.com/articles/fluid-viscosity-effects-on-valve-performance/
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

tudents are introduced to the similarities and differences in the behaviors of elastic solids and viscous fluids. In addition, fluid material properties such as viscosity are introduced, along with the methods that engineers use to determine those physical properties.
References
Soto-Castruita, Enrique et al. “Effect of the Temperature on the Non-Newtonian Behavior of Heavy Oils”. Energy & Fuels. (2015) Vol. 29, 5, pp. 2883-2889. https://doi.org/10.1021/ef502171d
Copyright
© 2024 by Regents of the University of Colorado; original © 2023 University of HoustonContributors
Duane Turner, North Shore Senior High School, Houston, TXSupporting Program
National Science Foundation GK-12 and Research Experience for Teachers (RET) Programs, University of HoustonAcknowledgements
This curriculum was developed under National Science Foundation RET grant no. 1855147— Research Experience for Teachers in Advanced Design and Manufacturing at the University of Houston. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Last modified: April 17, 2024



User Comments & Tips