Quick Look
Grade Level: 8 (7-8)
Time Required: 3 hours
(three 60-minute class periods)
Expendable Cost/Group: US $22.50 Many materials are reusable lab supplies and equipment; see the Materials List for details.
Group Size: 3
Activity Dependency: None
Subject Areas: Earth and Space, Science and Technology
NGSS Performance Expectations:

| MS-ESS3-3 |
| MS-ETS1-4 |
Summary
Students make sense of the problem with drinking water contamination due to various pharmaceuticals and hormones. They experience the steps of the engineering design process as they design solutions for a real-world problem that negatively affects the environment. Substances such as pesticides, prescription medication, and hormones are detected in the drinking water supplies of American and European metropolitan cities. Using chlorine as a proxy for estrogen and other drugs found in water, student groups design and test prototype devices that remove the contamination as efficiently and effectively as possible. They use plastic tubing and assorted materials such as activated carbon, cotton balls, felt and cloth to create filters with the capability to regulate water flow to optimize the cleaning effect. They use water quality test strips to assess their success and redesign for improvement. They conclude by writing comprehensive summary design reports while reflecting on how engineers can design solutions to help detect and effectively remove these contaminants from our drinking water.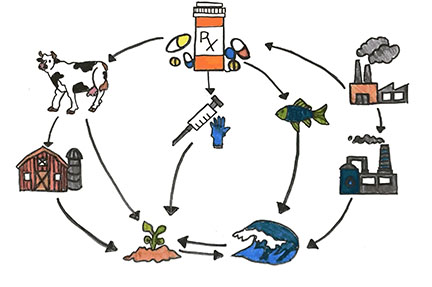
Engineering Connection
The water that comes from our faucets has passed through a complex system designed by many different types of engineers. Civil, chemical, electrical, environmental, geotechnical, hydraulic, structural and architectural engineers all play roles in the design, development and implementation of municipalities' drinking water supplies and delivery systems. Hydraulic engineers design systems that safely filter the water at a rate needed to supply a specific region and population. Chemical engineers precisely determine the amounts and types of chemicals added to source water for coagulation and decontamination to make the water safe to drink and not taste bad. Even so, modern pharmaceutical and agricultural practices persist in contaminating our water supplies. In this activity, students play the role of engineers challenged to design-build-test filtration devices to regulate flow and maximize output and decontamination.
Learning Objectives
After this activity, students should be able to:
- Discover a water quality-related problem and design possible solutions to solve the problem.
- Make sense of how pharmaceuticals and hormones have resulted in pervasive water contamination.
- Describe the effects humans have on the quality of water on Earth.
- Follow the steps of the engineering design process while designing and building prototype devices to filter contaminants from a liquid solution.
- Design a system that regulates flow rate to maximize effectiveness of water treatment.
- Filter out 75% of the chlorine from a provided chlorine solution "water pollutant"..
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ESS3-3. Apply scientific principles to design a method for monitoring and minimizing a human impact on the environment. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Apply scientific principles to design an object, tool, process or system. Alignment agreement: Construct and present oral and written arguments supported by empirical evidence and scientific reasoning to support or refute an explanation or a model for a phenomenon or a solution to a problem.Alignment agreement: | Human activities have significantly altered the biosphere, sometimes damaging or destroying natural habitats and causing the extinction of other species. But changes to Earth's environments can have different impacts (negative and positive) for different living things. Alignment agreement: | The uses of technologies and any limitations on their use are driven by individual or societal needs, desires, and values; by the findings of scientific research; and by differences in such factors as climate, natural resources, and economic conditions. Thus technology use varies from region to region and over time. Alignment agreement: Cause and effect relationships may be used to predict phenomena in natural or designed systems.Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-4. Develop a model to generate data for iterative testing and modification of a proposed object, tool, or process such that an optimal design can be achieved. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to generate data to test ideas about designed systems, including those representing inputs and outputs. Alignment agreement: | Models of all kinds are important for testing solutions. Alignment agreement: The iterative process of testing the most promising solutions and modifying what is proposed on the basis of the test results leads to greater refinement and ultimately to an optimal solution.Alignment agreement: | Models can be used to represent systems and their interactions. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of engineering design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop abilities to apply the design process.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the effects of technology on the environment.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Develop a solution to a technological problem that has the least negative environmental and social impact.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Massachusetts - Science
-
Describe and explain the purpose of a given prototype.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Identify and explain the steps of the engineering design process, i.e., identify the need or problem, research the problem, develop possible solutions, select the best possible solution(s), construct a prototype, test and evaluate, communicate the solution(s), and redesign.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Document and present solutions that include specifications, performance results, successes and remaining issues, and limitations.
(Grades
9 -
10)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- One 1.5 foot (0.5 m) plastic tubing or PVC pipe with 1.5 inch (3.81 cm) or greater inner diameter; available at hardware stores (see Figure 1, which shows an example tube that is longer than 1.5 feet; it is recommend to cut tubes to 1.5 feet to avoid difficulties in removing filter materials)
- Two 250 ml beakers
- 50-100 ml chlorinated water; this 200 ppm solution is prepared by the teacher using Clorox® germicidal bleach; see instructions in the Procedure section
- 50 ml graduated cylinder
- Plastic spoon, to load sands and activated carbon
- Sieve, to separate small materials
- Lab safety gloves, one pair per student per day, such as disposable nitrile gloves available at Amazon
- Lab book or notebook, one per student; alternatively, have students record all necessary information throughout the activity on blank sheets of paper and staple them together to serve as a lab "book" for the activity
- Safety goggles, enough so that each student in a team can wear goggles while handling and testing the chlorinated solution
- Engineering Design Report Scoring Rubric, one per student
- Claim-Evidence-Reasoning Graphic Organizer, two per student
To share with the entire class:
- Activated carbon, either granules or pellets; for cleanup, pellets are easier to separate with a sieve at activity end; see material life expectations note, below
- Filter media, such as cotton balls, fish filter media, carbon infused filter media, 50 micron felt pad, cheesecloth, cotton cloth or whatever is available
- Fine-grained sand
- (for teacher use only!) Clorox® germicidal bleach; used to make a chlorinated water solution; 2 teaspoons bleach per gallon of water
- Pitcher or other container large enough to hold a gallon of water, for preparing the chlorinated water
- "Free" and total low-level chlorine water quality test strips, such as Hach's "Free & Total Chlorine Test Strips, 0-10 mg/l," 50 strips, 0-10 ppm
- Sanitizer-strength chlorine test strips, such as LaMotte's "4250-BJ sanitizer strength chlorine test strips," 200 strips, 0-200 ppm, from Cole-Parmer
- Stopwatch
- Sink, tap water, soap, towels
Note: A pound of activated carbon can remove 200 ppm chlorine from 843 gallons, as calculated from https://www.waterprofessionals.com/learning-center/dechlorination/. Thus, a supply of activated carbon is reusable for several years of experiments.
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/wpi_protect_activity1] to print or download.Pre-Req Knowledge
Students should have prior knowledge of the water cycle (ESS2.C) and how increases in consumption of natural resources impact Earth's systems (MS-ESS3-4). Refer to the Natural and Urban "Stormwater" Water Cycles lesson for review on the water cycle and the You Are What You Drink! lesson for information on how drinking water is treated and distributed. It is important for students to have an understanding of the basic steps of the water treatment system to be able to comprehend that contaminants can result in our drinking water even after undergoing treatment. This will help students with figuring out a solution to the problem in the following activity.
Introduction/Motivation
"Who drank a glass of water today?" (see raised hands) "Does anyone know what is in a glass of water?"
Allows students to share their thoughts.
Tell students to draw a T-Chart in their notebooks. Label the first column “What I Notice” and the second column “What I Wonder”. Show students the following image and have students write down what they notice (observations) and wonder (questions) about it:

Allow students to share their thoughts out loud about what they noticed in the image. (Example: Pills coming out of a faucet and into a glass)
Now ask students to share what questions they have. (Example: Why are there pills coming out from the faucet? Where did the pills come from? What kind of pills are they? What does this mean? Is it harmful to us?) Write down student questions on the board. Tell students we are going to answer some of these questions today.
Guide student discussion to have students think about what causes the pills to end up in the cup and what effect that might have. Encourage students to share their thoughts with the class and discuss collaboratively.
Show students the following video: https://www.youtube.com/watch?v=XKCpeflOIUw
Now show students the following infographic:
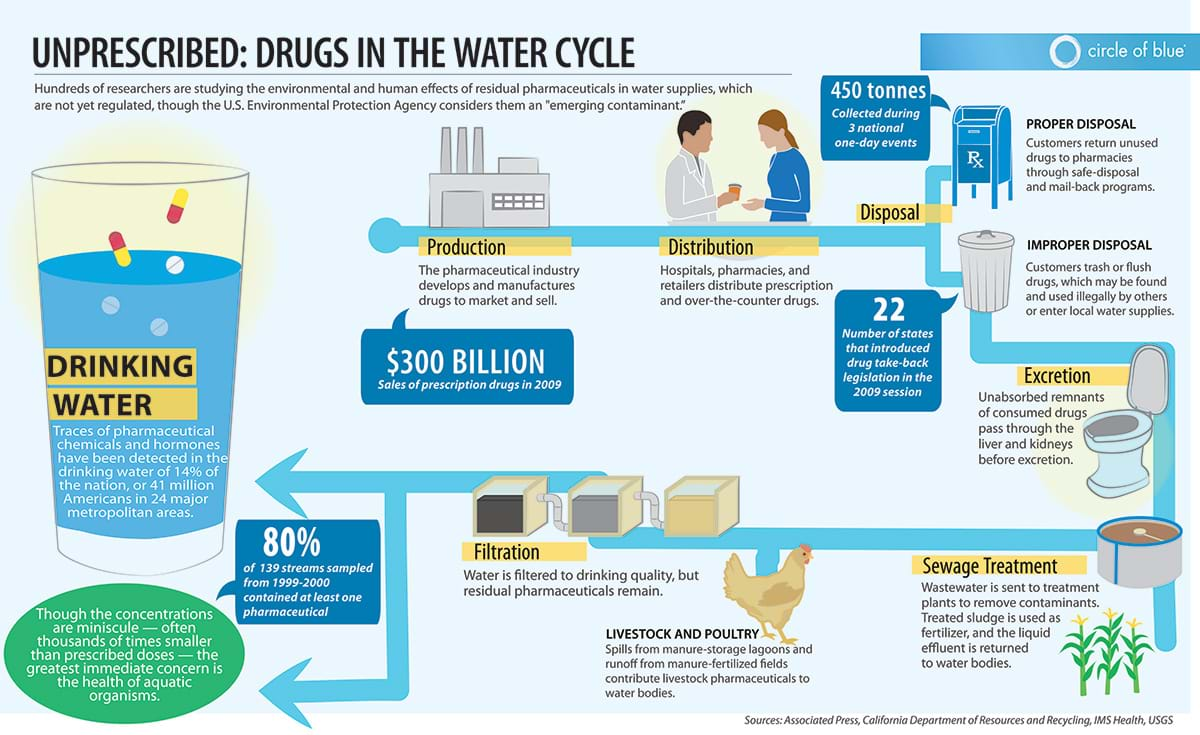
Tell students to first individually think about what problem is being presented (in the video and/or infographic). Have students identify what causes and effects result from this problem. (Example: The improper disposal of pharmaceuticals by humans results in traces of pharmaceuticals in our drinking water which negatively affects the health of aquatic organisms.) Encourage students to share their thoughts in small groups first and then come together for a large classroom discussion.
During student discussion, guide students to notice that pharmaceutical chemicals still appear in drinking water even after it goes through water treatment and filtration.
For additional information on the problem, students can listen to USGS's podcast on Emerging Contaminants, Pharmaceuticals in S. Carolina Rivers & Streams. The transcript of the podcast is also available if students prefer to read. (Note: This resource can also be used for the research section of the Engineering Design Process described later in the activity.)
Note to teachers: Find and share information about contaminants in your local waterways to help students better relate. The EPA's How's My Waterway? interactive application is a great tool to learn about your community's water quality, including any drinking water contaminants. The EPA also provides access to all local Consumer Confidence Reports (the annual drinking water quality report from your water supplier) which indicates the source of water and what's in it, including any levels of contaminants and the EPA's health-based standard (maximum contaminant level) for comparison.
Tell students, that as all the examples above suggest, our water waste-treatment facilities aren’t always designed to get rid of pharmaceutical compounds and hormones, allowing these contaminants to remain in our local waterways and even the water we drink.
To assess student understanding thus far, have students fill out the Claim-Evidence-Reasoning Graphic Organizer to answer the question:
How does the human activity of improper disposal of pharmaceuticals affect the environment and impact living things?
Encourage students to use the sentence starters provided in the organizer to write their responses. Students can refer to the second page of the graphic organizer for help with writing their reasoning. (Note: For lower grades, consider completing this as a class as described in the Activity Scaling.)
Once complete, ask students to share their responses with the class. As students share their responses, encourage students to respectfully agree/disagree with others' responses and ask them to explain why/why not. (For example, the teacher can ask a student "Do you agree with what ___ said?" "Why or why not?") Refer to the Claim-Evidence-Reasoning Graphic Organizer Example 1 to ensure student discussion is on track. If students are struggling, refer back to the video and/or infographic.
Once all students have a good understanding, tell students:
"Now that you are familiar with the problem, what question(s) do you have?" Encourage students to revisit their questions they wrote down at the beginning. Listen to student responses, guiding students to ask what solutions might exist to help remove pharmaceuticals and other organic compounds from the water.
"So, what solutions exist to help remove these contaminants from our drinking water and just how effective are they?"
Give students time to brainstorm solutions to the problem and what they could do in order to solve it. Have them think about how a potential model would look, what the different components of the system would be and how the components would interact with each other.
Encourage students to first discuss their thoughts in small groups and then share out to the whole class. Write down their suggested solutions on the board and ask students to discuss how successful they think each solution would be in helping to solve the problem. During student discussion, help guide students towards the solution of creating a filtration device if students don't on their own (i.e., Ask students: "What about some sort of filtration device? Could that work?").
After students share their thoughts, present them with the design challenge:
"Today, acting as an environmental engineer, your challenge is to design a filtration device that could be implemented in your local water treatment system to effectively remove estrogen and other organic compounds from drinking water as a final step in the filtration system."
Procedure
Background
Activated carbon (or charcoal) and sand filtration are commonly used to remove particles, organic matter, bacteria and pathogens from water. To keep aquariums healthy for fish, filters with activated carbon are employed to remove tap water chemicals and the chemicals produced from fish waste. Typically, municipal water treatment facilities do not use activated carbon in their filtration processes in order to reduce cost and maintenance. However, activated carbon reduces the amount of estrogen in drinking water by 60-80%.
Provide students with research (see the References section) that documents so they recognize themselves of the successful use of activated carbon to remove estrogen, the disinfectant that is added to water and other harmful pollutants that may be present in drinking water. For multilingual learners, consider providing research articles in a different language (Note: The References section contains two articles in Spanish that can be used).
Before the Activity
- Gather materials and make copies of the Engineering Design Report Scoring Rubric and the Claim-Evidence-Reasoning Graphic Organizer.
- Depending on available supplies, set a maximum material amount that can be used by each group. For example, if you have 40 ounces of activated carbon available and 10 groups, set a 4 oz. maximum per team. If you only have one 24 x 24-inch square of felt, then each group may use a maximum of 2.4 x 2.4-inch square.
- Prepare the chlorine solution: Pour 2 teaspoons of Clorox® germicidal bleach into 1 gallon of water to create a 200 ppm solution.
With the Students
Day 1
- Present the Introduction/Motivation content to the class.
- Review lab safety with students. Inform them that they will be using a bleach solution. Ask them to tell you what lab safety procedures apply to this situation. Refer to the guidelines suggested in the Safety Issues section. Remind students to wear lab gloves and safety goggles when handling and testing the chlorinated water.
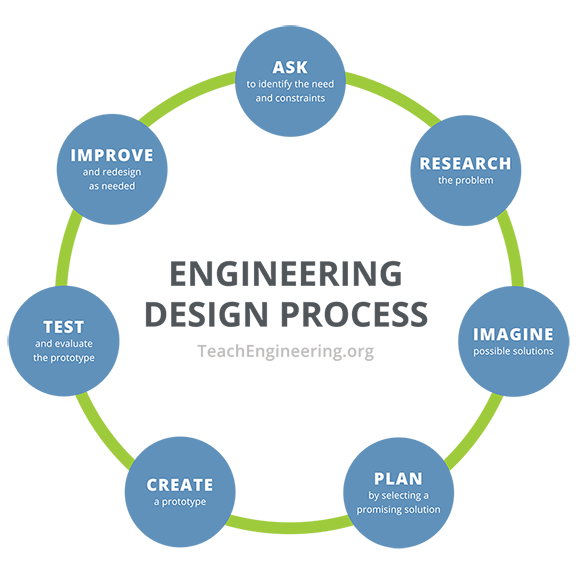
- As a class, review the steps of the engineering design process. Refer to https://www.teachengineering.org/PDF/edp/TE_EDPTeacherMaterials_8.5x11.pdf. The basic steps are: Identify the need and constraints, research the problem, develop possible solutions, build a prototype(s), test and evaluate the prototype(s), redesign as needed.
- Divide the class into groups of two or three students each. Or, if materials are scarce, organize the class into groups of three or four students each.
- Review the rubric with students so they understand the activity expectations and the assessment criteria. Inform them of the available supply of materials and any per group material limitations.
- Have students spend some time identifying the criteria and constraints given the problem within their groups. (Example: device needs to ensure water is visibly clearer, materials constraint, etc.) Have students write their ideas in their lab book/journal.
- Explain to the class how activated carbon can attract and trap estrogen, organic compounds and chlorine. As water travels through students' prototype filter designs, it is important to be able to control the flow of the water in order to give the activated carbon time to remove the toxins. If the water goes through the activated carbon too fast, it will not purify the water because not enough contact time is provided; if water goes through the carbon too slowly, then the prototype is not efficient for practical use. In situations like this, engineers must find the balance that is most suitable for the purpose and goals of the device being designed.
- Inform students that they will be required to take apart their prototypes at the end of class and separate all materials. A sieve will be provided to separate any mixed sand and activated carbon. Other materials such as wool, filter media and felt will also be separated and returned to their designated storage spots.
- Do a few water quality tests in front of the class to demonstrate how to test water and read the results. First, use a 0-200 chlorine test strip to test the chlorinated solution that was made for the class. The solution should be 200 ppm chlorine. Then test the school's tap water, as taken from a drinking fountain or sink tap, using both the 0-200 chlorine test strip and the 0-10 chlorine test strip so you can compare the school's drinking water to the highly chlorinated water of the pre-made solution. Show students the test strips and how to compare their colors to the scale provided with each type.
- Give groups their filters (the tubes) and let them begin the design process. Remind students that they will be creating models to represent a water filtration system.
- Allow students to engage in the second step of the design process by conducting online research. The References section provides some articles they can use to better understand the problem and the role of activated carbon in water filtration. (Note: For lower grades, consider completing this as a class as described in the Activity Scaling.)
- Direct groups to start by brainstorming at least three different designs keeping in mind the criteria and constraints they previously discussed. Students could choose to describe their designs by talking about it within their group, drawing it and/or explaining it in writing. Require (at least) one team member in each group to take notes and document all ideas from the brainstorming session, including sketching out various design ideas. Mention to students that throughout all the steps of the design process, engineers benefit from taking detailed notes of all their ideas and test results, including sketches.
- After groups have brainstormed multiple designs, direct them to discuss the merits of each design, comparing them to each other in order to select the most-promising design (or combine various ideas into one most-promising design) given the criteria and constraints they previously discussed. Next, have the teams create more detailed sketches of the chosen designs; make sure these drawings are labeled with dimensions/amounts and materials. Remind students to write down the reasoning behind the final design and each material selection, as well as how each is intended to function.
As students are working, assess their ideas by asking some of the following guided questions:
- What are you trying to accomplish in the design of your water filtration device?
- How does your prototype model represent a water filtration system?
- What are they key parts of the filtration device and their purpose? How do the different components of the device interact together?
- How does your device satisfy each of the criteria and constraints determined earlier?
- What are different ways to use the materials provided in your filtration device?
For groups that are struggling, spend some time listening to their team discussion and answering any questions they might have.
- Have each team share their designs with another team and provide feedback on each other's designs. Allow students to revise their designs based on team feedback if needed.
- Check each design sketch before permitting students to gather the necessary materials.
- Once teams have acquired their materials, direct them to begin creating their prototypes.

Figure 1. One of the first prototypes! After this, tubes were cut shorter, to 1.5-foot lengths, after unloading proved to be difficult. - When 15 minutes are remaining in the period, direct students to test their current designs and come up with solutions to any problems they may want to fix during the next day.
- Testing procedure:
- A group brings its prototype up to the teacher. They explain their design concept and the purpose of each layer in their filter prototype.
- Then students hold the prototype vertically above a 250-ml beaker while the teacher pours 50 ml of 200 ppm chlorinated water into the prototype.
- One student uses a stopwatch to record the time it takes for the water to move through the filter.
- Once the liquid has stopped dripping out of the bottom of the prototype (to a rate of about one drip every five seconds), the teacher uses a chlorine test strip to test the dripping water to determine if students have achieved the goal of lower than 50 ppm chlorine. If they have, then the teacher uses a 0-10 ppm chlorine test strip to determine the best estimate of the chlorine concentration of the water.
- Students also measure the amount of water retrieved from the filter.
- In their lab notebooks, students record the chlorine concentration, the time it took the water to move through the filter, and the amount of water retrieved from their filters.
- Direct the team to spend some time making notes in their notebook about the "lessons learned" from the prototype testing. What worked? What did not work? What could be improved? What new ideas do you have? Analyze and interpret data to determine similarities and differences in findings. Ask students to identify limitations of the use of their device to solve the problem.
- With five minutes left of the class period, have students disassemble their prototypes and separate all materials into piles of like materials so they are ready to be used for the next class.
Day 2
- Direct students to redesign their filter prototypes with the same expectations of the previous day. This means that groups can modify or completely re-design their water filtration systems from Day 1. Encourage teams to share their ideas with other groups to receive feedback.
- Allow teams to make improvements based off team feedback and from Day 1's testing.
- When teams have completed their revised prototype designs, they take them to the teacher and explain the purpose of each layer of the filter prototypes.
- The teacher can provide each group with feedback on their designs and then allow student groups time to make modifications based on teacher feedback.
- With the teacher's help, groups test their (hopefully) improved prototypes. They measure the time it takes for water to pass through the system, the after-filtered chlorine concentration with the chlorine test strips, and the amount of water that was retrieved, as they did the previous day. Students should record all results in their lab notebooks.
- Direct students to answer the Guiding Questions in their lab books/journals, as provided in the Assessment section. Collect their lab books for grading.
- Direct students to begin individually writing up their observations and results into final engineering design reports, as described in the Assessment section. Give them a deadline to turn them in and grade them with guidance from the rubric. Students may instead be given the option to present their observations and results any way they wish (i.e., draw a picture, make a video).
- Have students complete a new Claim-Evidence-Reasoning Graphic Organizer, as described in the Assessment, that answers the question: What solutions exist to help remove pharmaceutical compounds from our drinking water and are they effective? (Note: For lower grades, consider completing this as a class as described in the Activity Scaling.)
- Allow students to present their CER along with their designs to the class. Tell students they should imagine they are pitching their solution to their local water treatment facility.
- Groups disassemble their filtration systems and separate the materials into piles for the next class or for storage.
Vocabulary/Definitions
aeration: Mixing air with a substance.
anthropogenic: Caused or produced by humans.
coagulation: The clumping of a material into larger particles. Example: Blood coagulates to stop a cut from bleeding.
estrogen: A hormone that is naturally found in the human body. This hormone maintains female characteristics of the body and is replicated in birth-control pills.
pharmaceutical: A drug that is used for medical reasons; may be available over-the-counter or prescribed by a doctor.
prototype: A first attempt or early model of a new product or creation. May be revised many times
sedimentation: The process of settling. Example: Particles settle to the bottom of a water column.
Assessment
Pre-Activity Assessment
Claim-Evidence-Reasoning: Have students complete the Claim-Evidence-Reasoning Graphic Organizer to answer the following question:
- Describe how humans impact and affect the environment and living things when we do not properly dispose pharmaceuticals, such as putting medications down sink drains, toilets, or in the trash.
Students can refer to the second page for help with writing their reasoning. Refer to the Claim-Evidence-Reasoning Graphic Organizer Example 1 for an example of a student response.
Activity Embedded (Formative) Assessment
Lab Book/Journal: Individually assess students based on the design and redesign documentation in their lab books or journals. Require each student's lab book to contain design ideas, prototype sketches and a description of the functionality and results from prototype testing. Expect students to include observations and hypotheses about their prototype designs and material use. Direct them to document their reasoning for each design they built and tested. For full credit, require students to answer the following Guiding Questions for the design portion of the lab book:
- What are you trying to accomplish in the design of your water filtration device?
- What are different ways to use the materials provided in your filtration device?
- How does your prototype model represent a water filtration system?
- What are they key parts of the filtration device and their purpose? How do the different components of the device interact together?
- How does your device satisfy each of the criteria and constraints determined earlier?
- How can you control water flow to enable the chlorinated water to react with the activated carbon?
- What are the results of your water filtration device's test?
- In what step of the municipal water treatment plant filtration process would you implement your design?
Post-Activity (Summative) Assessment
Summary Engineering Design Reports: Students prepare and hand in engineering reports on their design work completed on both days, including the pros and cons of their designs. Grade students on their ability to critique their designs and their improvements from Day 1 to Day 2 and suggestions to further improve the prototypes. Students whose designs did not improve can still obtain high-achieving grades by thoroughly explaining the ways that their second designs did not work compared to their first designs and how they can further improve their designs. Use the Engineering Design Report Scoring Rubric for guidance in assessing students' reports.
Claim-Evidence-Reasoning: Have students complete a new Claim-Evidence-Reasoning Graphic Organizer by now answering the following question:
- What solutions exist to help remove pharmaceutical compounds from our drinking water and are they effective?
Refer to the Claim-Evidence-Reasoning Graphic Organizer Example 2 for an example of a student response. Allow students to present their CER along with their designs to the class. Tell students they should imagine they are pitching their solution to their local water treatment facility.
Making Sense: Have students reflect on the engineering problem they explored and the science and engineering skills they used by completing the Making Sense Assessment.
Safety Issues
Good lab practices for this activity:
- Wear safety goggles and gloves when handling and testing the chlorine solution and filter materials (because they are re-used and thus contain the chlorine solution).
- Bleach products at this level of concentration may cause skin irritation. If any chlorine solution gets in your eyes or mouths, immediately flush the area with water for 10 minutes.
- If any solution gets on your skin, wash the skin with water and soap for five minutes.
- If any solution spills on a desk or the floor notify the teacher so the spill can be cleaned up.
Activity Extensions
If time permits, consider having students scale the best design in the class to their local full-scale water treatment system. For example, a full-scale water treatment system can filter 4.5 million gallons per day, which is the city of Marlborough, MA's average daily water use.
Have students research their local community's water treatment facility and determine what water treatment methods are used. Plan for a site visit to the facility if possible.
Activity Scaling
For lower grades, complete the Claim-Evidence-Reasoning Graphic Organizer together as a class. Go over the research step of the engineering design process as a class. Additionally, the Summary Engineering Design Report can be optional.
For advanced students, have students research other effective methods to filter water. Challenge students to come up with an argument for how their researched method compares to the use of activated carbon. Additionally, students can brainstorm how socio-economically disadvantaged areas can clean their water on a limited budget and present their ideas to the class.
For multilingual learners, consider providing research articles in a different language. For example, the following are great articles in Spanish that can be used for research:
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are challenged to think as biomedical engineers and brainstorm ways to administer medication to a patient who is unable to swallow. They learn about the advantages and disadvantages of current drug delivery methods—oral, injection, topical, inhalation and suppository—and pharmaceutical desi...
References
Doheny, Kathleen. "Drugs in Our Drinking Water? Experts Put Potential Risks in Perspective After a Report that Drugs Are in the Water Supply." Published March 10, 2008. WebMD Feature, WebMD. Accessed July 2, 2014. http://www.webmd.com/a-to-z-guides/features/drugs-in-our-drinking-water
Rowsell, Victoria F., Dawn S.C. Pang, Foteini Tsafou and Nikolaos Voulvoulis. "Removal of steroid estrogens from wastewater using granular activated carbon: comparison between virgin and reactivated carbon." Water Environment Research. April 2009, 81(4): 394-400. Print.
GRDULSKA, AGNIESZKA; KOWALIK, ROBERT. (2020, September). (PDF) estrogen removal from wastewater. Retrieved September 21, 2021, from https://www.researchgate.net/publication/348645180_ESTROGEN_REMOVAL_FROM_WASTEWATER.
Rojas Rojas, M., Castellanos Ballesteros, J., & Castañeda Jaimes, L. (2021, January 01). Revisión de literatura científica sobre factores predisponentes de conductas de abuso sexual infantil en hombres colombianos. Retrieved February 03, 2022, from https://repository.ucc.edu.co/handle/20.500.12494/33198?mode=full
DiCYT, A. (n.d.). UN Filtro de Tela de Carbón Activado y bacterias elimina compuestos Químicos del Agua. Retrieved February 03, 2022, from https://www.dicyt.com/noticias/un-filtro-de-tela-de-carbon-activado-y-bacterias-elimina-compuestos-quimicos-del-agua
Copyright
© 2015 by Regents of the University of Colorado; original © 2014 Worcester Polytechnic InstituteContributors
Timothy S. Vaillancourt, Terri Camesano, Kristen Billiar, Jeanne Hubelbank; Dua ChakerSupporting Program
Inquiry-Based Bioengineering Research and Design Experiences for Middle-School Teachers RET Program, Department of Biomedical Engineering, Worcester Polytechnic InstituteAcknowledgements
This activity was developed under National Science Foundation RET grant no. EEC 1132628. However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: May 18, 2023



User Comments & Tips