Quick Look
Grade Level: 8 (7-9)
Time Required: 45 minutes
Expendable Cost/Group: US $6.00 The activity uses non-expendable (and reusable) Brita water pitchers; see the Material's List for details.
Group Size: 3
Activity Dependency:
Subject Areas: Chemistry, Measurement, Physical Science, Problem Solving, Reasoning and Proof, Science and Technology

Summary
Students measure the effectiveness of water filters in purifying contaminated water. They prepare test water by creating different concentrations of bleach (chlorine-contaminated) water. After passing the contaminated water through commercially available Brita® water filters designed to purify drinking water, students determine the chlorine concentration of the purified water using chlorine test strips and measure the adsorption of chlorine onto activated carbon over time. They graph and analyze their results to determine the effectiveness of the filters. The household active carbon filters used are one example of engineer-designed water purification systems.Engineering Connection
Environmental and chemical engineers add small amounts of chlorine to water during treatment processes because chlorine is a known disinfectant. However, too much chlorine in drinking water can result in adverse health effects for humans. A remedy to remove excess chlorine and other contaminants from drinking water is activated carbon, which is charcoal designed by engineers for both large and small scale water purification use. Understanding the adsorption of contaminants in systems such as activated carbon over an extended time period is vital for environmental engineers to produce more efficient and safe purification models.
Learning Objectives
After this activity, students should be able to:
- Apply their engineering knowledge to a commercially available water purification system.
- Describe how activated carbon in water purification filters removes contaminants.
- Determine the efficiency of household water purification systems in chlorine removal and apply that knowledge to other contaminants.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Models can be used to represent systems and their interactions—such as inputs, processes and outputs—and energy and matter flows within systems.
(Grades 6 - 8)
More Details
Do you agree with this alignment?
-
Construct and interpret graphical displays of data to identify linear and nonlinear relationships.
(Grades 6 - 8)
More Details
Do you agree with this alignment?
-
Conduct an investigation to produce data to serve as the basis for evidence that meet the goals of an investigation.
(Grades 6 - 8)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Model with mathematics.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Construct and interpret scatter plots for bivariate measurement data to investigate patterns of association between two quantities. Describe patterns such as clustering, outliers, positive or negative association, linear association, and nonlinear association.
(Grade
8)
More Details
Do you agree with this alignment?
-
Investigate patterns of association in bivariate data.
(Grade
8)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
The management of waste produced by technological systems is an important societal issue.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Analyze how the creation and use of technologies consumes renewable and non-renewable resources and creates waste.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Assess a technology that minimizes resource use and resulting waste to achieve a goal.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Missouri - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Model with mathematics.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Construct and interpret scatter plots for bivariate measurement data to investigate patterns of association between two quantities. Describe patterns such as clustering, outliers, positive or negative association, linear association, and nonlinear association.
(Grade
8)
More Details
Do you agree with this alignment?
-
Investigate patterns of association in bivariate data.
(Grade
8)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Missouri - Science
-
Organisms are interdependent with one another and with their environment
(Grades
K -
4)
More Details
Do you agree with this alignment?
-
The nature of technology can advance, and is advanced by, science as it seeks to apply scientific knowledge in ways that meet human needs
(Grades
K -
5)
More Details
Do you agree with this alignment?
-
Human activity is dependent upon and affects Earth's resources and systems
(Grades
6 -
7)
More Details
Do you agree with this alignment?
-
Science understanding is developed through the use of science process skills, scientific knowledge, scientific investigation, reasoning, and critical think
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- Brita water filter and corresponding Brita water pitcher (use a ten 8-ounce cup model Brita water filter in order to hold the necessary amount of water for this lab; to run the activity again, re-use the pitchers and buy replacement filters at $8 each)
- Hydrochloric acid (HCl) solution from Sigma Aldrich (0.1 M; available at https://www.sigmaaldrich.com/catalog/substance/hydrochloricacidsolution3646764701011?lang=en®ion=US)
- 1-liter beaker
- 17 paper cups (30-ounce or ~0.90-liter in volume)
- 17 LaMotte chlorine test strips, 10–200 ppm
- tap water
- large bowl, in which to activate filters
- pipette
- duct tape, enough to wrap around and secure the filter lid to the pitcher base
- stopwatch
- safety eye goggles, one per student
- baking soda (sodium bicarbonate powder), 1 box (16-oz, 454 g) to neutralize solutions before disposal
- Remediation Lab Worksheet, one per student
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/wst_environmental_lesson02_activity2] to print or download.Pre-Req Knowledge
Have students complete the Introduction to Water Chemistry lesson before conducting this activity.
Introduction/Motivation

Earlier we talked about how contaminants and pollutants enter groundwater. We also talked about how engineers design ways to clean groundwater and make it safe to drink. Running water through activated carbon (show Figure 1) adsorbs contaminants and allows clean water to pass through.
Activated carbon is charcoal that has been treated with oxygen to open up millions of tiny pores between the carbon atoms. The manufacturing process results in highly porous charcoals that have surface areas of 300 to 1,000 square meters per gram! This "activated" charcoal is widely used to adsorb smelly or colored substances from gases or liquids.
What do we mean by "adsorb"? When a material adsorbs something, it attaches to it by chemical attraction. The huge surface area of activated charcoal gives it countless bonding sites. When certain chemicals come near the carbon surface, they attach to it and are trapped. Activated carbon is good at trapping other carbon-based impurities, as well as things like chlorine, while many other chemicals are not attracted to carbon at all (sodium, nitrates), so are unaffected by the activated carbon and pass right through. So, activated charcoal filters remove certain impurities while ignoring others.
In today's lab, we are going to see how well Brita water filters, which use activated carbon, remove chlorine from bleach water. Chlorine is used as a disinfectant in treating drinking water, but too much is dangerous to humans; it can damage your nervous system and may cause cancer.
Procedure
Before the Activity
- Gather materials and make copies of the Remediation Lab Worksheet.
- Set up each lab station with a Brita filter and pitcher. Allow 15 minutes or so to activate the filter, following the instructions that come with the filter. Once activated, seat them in the Brita water pitchers.
- Have each lab group test different concentrations of chlorine-water. We recommend testing in the range of 50–200 ppm. Refer to Table 1 to prepare 1 L of each of the Cl solution to be tested by students. Use pipettes to accurately prepare the solutions.
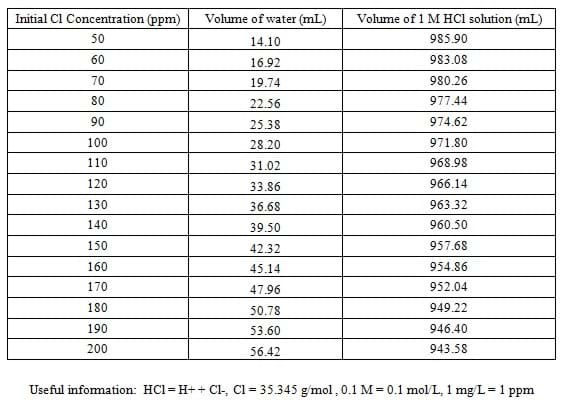
Table 1. The volumes of water and 1 M HCl solution necessary to create the desired concentrations of chlorine-water for the lab.
With the Students
- Divide the class into groups of three students each.
- Hand out the worksheets and assign each group an initial chlorine concentration.
- Prepare the chlorine-contaminated water by following Table 1 to add a certain volume of water and 1 M HCl in the beaker. Perform the calculations with your students; see instructions in Figure 2.
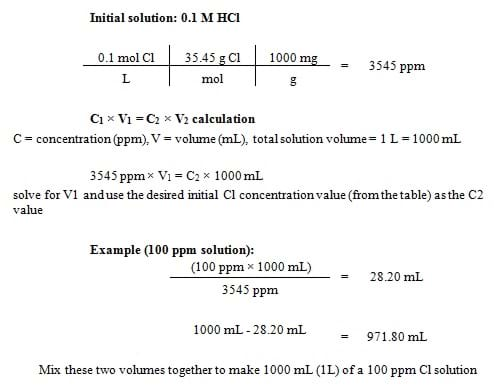
Figure 2. Example calculations to determine how much water to add to an initial solution of 0.1 M HCl to create 1 liter (1000 mL) of a 100 ppm chlorine solution. - Once the sample chlorine-water is made, use a chlorine strip to test the starting concentration of the contaminated water. Do this by pouring a small sample of the beaker water into a paper cup. Dip the test strip in the water sample and then compare the test strip to the scale provided in the test strip vial to determine the chlorine concentration. Record the chlorine concentration in the worksheet table—this is the measurement corresponding to time 0. IMPORTANT: Remind students to never reuse a paper cup or test strip; always use a new paper cup and test strip for each sample.
- Pour the chlorinated water into the opening at the top of the Brita filter lid to begin collecting it in the pitcher base. If the full liter of chlorine solution does not fit, then just add enough of the solution so that the top reservoir of the water pitcher (containing the solution to be filtered) is full. Add the rest of the solution when enough room frees up.
- Two minutes after the chlorine-contaminated water has started passing through the filter to be collected in the pitcher base, use another chlorine strip to test the concentration of the water. Do this by pouring a small sample of the filtered solution in the base of the Brita pitcher into a paper cup and take a chlorine measurement, just as in step 4. Record the results in the worksheet table.
- Every two minutes, pour another small sample of filtered water into a new paper cup and use a new chlorine strip to test the concentration. Record the concentrations in the table after each sampling. Continue to take measurements for 30 minutes.
- After data collection is complete, graph the chlorine concentration vs. time to see the adsorption pattern.
- Once the graph is complete, pour water straight from a tap into a paper cup. Use a chlorine strip to measure the concentration of the chlorine. Record this measurement.
- Leave the chlorinated solutions at your station to be disposed of correctly by the instructor.
- Have students answer the questions on their worksheets and hand them in for grading.
- Conclude by leading a wrap-up class discussion to compare results and conclusions; see questions in the Assessment section.
Vocabulary/Definitions
activated carbon: Processed carbon that is extremely porous, providing great amounts of surface area to allow for high adsorption. Also called activated charcoal.
adsorption: The binding of atoms, ions or molecules from a gas, liquid or dissolved substance to a surface. Such as on the surfaces of pores of activated carbon.
contamination: The presence of a minor component in another chemical or mixture.
remediation: To correct something that has gone bad or defective; to provide a remedy.
Assessment
Pre-Activity Assessment
Lesson Recap: As a class, review the concepts explored in the associated lesson. Focus the conversation on ways people purify their drinking water sources and how activated carbon works. Ask students if they know of any chemicals intentionally added to drinking water (such as chlorine and fluorine).
Activity Embedded Assessment
What's Going On? While students are conducting the lab, with guidance from the Water Remediation Lab Worksheet, walk around and ask them questions to keep them engaged and on task. Example questions:
- Why would we find chlorine in our drinking water? Is it safe? (Answer: It may surprise some students to learn that chlorine is added to drinking water during the municipal water treatment process for the purpose of disinfecting the water. Basically, it kills any microbes in the water that could be dangerous. In very small concentrations, chlorine is not considered to be harmful, but in large concentrations it can be poisonous. The EPA regulation for chlorine is 4 mg per liter or 4 ppm.)
- What could be some sources of excess chlorine? (Answer: One class of chemicals exists that are "chlorinated" [that is, they have chlorine atoms in their chemical structures] and are usually toxic to animals [including humans] when ingested. Most sources of these chlorinated compounds arise from byproducts of industrial applications. For example polychlorinated biphenyls [PCBs] are compounds that have been used as coolants for old light systems and exist in high concentrations in some drinking water sources.)
- Where would be an example of a place that we purposely add excess chlorine to water? What does that water taste like? Is it safe to drink? (Answer: Swimming pools are an example of where we might add excess chlorine to water. This water is not meant for drinking! It has a strong taste and smell of chlorine. If you have been to a swimming pool and smelled the pool water or tasted it, then you have experienced it. On the other hand, the U.S. and other countries have purposefully added chlorine to drinking water because chlorine is a good disinfectant. Low concentrations are generally considered safe to drink; however, at some point, negative health effects occur. In the U.S., a limit is set by the Environmental Protection Agency (EPA). If the concentration of chlorine in water is above the EPA regulation, then it is considered unsafe to drink.)
- Do you expect the chlorine concentration during the middle of the filtration experiment to be the same, higher or lower than the measurement at the end? (Answer: This may vary, but it is expected that the concentration of chlorine in the middle of the experiment will be higher than at the end.)
Post-Activity Assessment
Worksheet: Have students answer the worksheet questions and hand them in for grading (see answers in the Wrap-Up Discussion, below). Review their answers to gauge their comprehension of the material.
Wrap-Up Discussion: At lab end, bring students together as a class and ask them the following questions about how well their activated carbon filters removed chlorine. Make sure everyone understands the answers and how they relate to groundwater contamination and remediation
- How well did the water filter separate the chlorine from our contaminated water? Look at your graphs for help. (Answers will vary, depending on the starting concentrations. For groups with higher initial chlorine concentrations, high levels of chlorine may remain in the filtered water. For groups that started with lower initial concentrations, expect to see almost complete chlorine removal. To answer the question, have students describe the measurements they took and the graphs they created. Have them comment on whether their data indicated that chlorine was being removed from the water or if the filter was no longer removing chlorine.)
- The amount of chlorine permitted in drinking water set forth by the Environmental Protection Agency is 4 ppm. Was the "contaminated water" we tested in this activity safe to drink before filtration? After filtration? Is our tap water safe to drink? (Answer: The water we tested in this activity would not be safe to drink as different groups began with concentrations ranging from 50–200 ppm chlorine. Expect the drinking water from the tap to be safe to drink. We tested this using test strips, although our test strips had a detection limit of 10ppm, meaning we can only measure if the chlorine concentration is below 10 ppm. If we found that the tap water has a chlorine concentration below the detection limit, we can assume that it is safe to drink.)
- How does activated carbon in water purification filters remove chlorine and other contaminants? (Answer: Activated carbon is a manufactured charcoal with a submicroscopic network of millions of tiny pores. The extremely large surface area per unit volume provided by this material gives it countless bonding sites to adsorb chlorine and other contaminants that pass nearby. When a material adsorbs something, it attaches to it by chemical attraction and traps it, making activated carbon a good remediation method to remove certain impurities from water.)
- From what you know about active carbon, will our Brita activated carbon filter last forever? (Answer: No. Remember that the nature of active carbon is that it has millions of tiny pores that provide an extreme amount of surface area on which the chlorine can bind. But, once all of the bonding sites are filled, an activated carbon filter stops working and you must replace it.)
- The Brita filter used in this activity has a standard thickness and shape. Using what you've learned from your breakthrough curves (chlorine concentration versus time), do you think that the thickness of the Brita filter was adequate for removing the chlorine from the water in your test? What about for groups that tested higher chlorine concentrations than you? If the chlorine concentrations of the samples you collected after filtration were too high (i.e. above the EPA standard), what would you change about the design of the Brita filter?
Safety Issues
- Beware of getting bleach on skin, eyes or clothing. Provide safety eyewear. Have the instructor pour the bleach to prepare the solutions.
- At experiment end, collect all the chlorinated solutions and neutralize them by adding baking soda (sodium bicarbonate powder). The neutralization is complete after bubbling has stopped. Once this is done, pour the solutions down the drain.
Troubleshooting Tips

For this lab, using Brita water pitchers works best. For some Brita pitcher systems, if you pour out a sample of the filtered water in the base while water is still present in the reservoir above the filter (waiting to be filtered), it causes the top to fall off and the water in the reservoir to pour out. You can avoid this mishap, and not lose the solution about to be filtered, by duct taping the top of the pitcher to the base, and only permitting students to pour the solution into the reservoir via the hole on the top piece. Figure 3 illustrates this suggestion.
In the concentration vs. time graphs, it is typical to see a high initial chlorine concentration followed by a gradual decrease. At first, the amount of chlorine that passes through the filter could be significantly decreased, or the same as the starting solution—it depends on how well the filter is activated (and also the effectiveness of the filter) before the experiment. Over time, expect the chlorine concentration to decrease. Near the end of the experiment, the concentration may level out (that is, no more chlorine removal) due to decreases in the filter efficiency (saturating the filter).
Activity Extensions
Repeat the lab using different chemical test strips, such as those that measure pH, copper, iodine or ammonia. Encourage students to repeat this lab with other chemicals and with the help of their parents to test the efficiency of home water purification systems on those chemicals, as well as testing well water or municipal tap water or swimming pool water.
Activity Scaling
- For lower grades, prepare the chlorine-contaminated water prior to the lab activity and guide students in creating the adsorption plot.
- For upper grades, have students determine their own chlorine concentrations to test based on the range of the chlorine test strips.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are presented with examples of the types of problems that environmental engineers solve, specifically focusing on water quality issues. Topics include the importance of clean water, the scarcity of fresh water, tap water contamination sources, and ways environmental engineers treat contamin...
References
What is activated charcoal and why is it used in filters? Last updated April 1, 2000. Science > Environmental Science > Energy Production: Question 209, HowStuffWorks.com. Accessed January 2, 2013. http://science.howstuffworks.com/environmental/energy/question209.htm
Copyright
© 2013 by Regents of the University of Colorado; original © 2010 Washington University in St. LouisContributors
Jessica Ray; Phyllis Balcerzak; Barry Williams; Carleigh SamsonSupporting Program
GK-12 Program, School of Engineering and Applied Science, Washington University in St. LouisAcknowledgements
This curriculum was developed with support from National Science Foundation GK-12 grant number DGE 0538541. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: April 8, 2025



User Comments & Tips