Quick Look
Grade Level: 8 (7-9)
Time Required: 45 minutes
Lesson Dependency:
Subject Areas: Chemistry, Physical Science, Science and Technology
NGSS Performance Expectations:

| MS-PS1-1 |
Summary
After students conduct the two associated activities, Density Column Lab - Parts 1 and 2, present this lesson to provide them with an understanding of why the density column's oil, water and syrup layers do not mix and how the concepts of density and miscibility relate to water chemistry and remediation. Topics covered include miscibility, immiscibility, hydrogen bonds, hydrophobic and hydrophilic. Through the density column lab activities, students see liquids and solids of different densities interact without an understanding of why the resulting layers do not mix. This lesson gives students insight on some of the most fundamental chemical properties of water and how it interacts with different molecules.Engineering Connection
For any source of contaminated water, environmental engineers must understand the physical properties and, especially, the chemical properties of contaminants so as to better understand how they interact with water molecules. An understanding of how liquids interact, explained by the science of molecular bonding, is pivotal to environmental engineering in terms of contaminated water remediation and water treatment to provide safe drinking water.
Learning Objectives
After this lesson, students should be able to:
- Define the properties of miscibility and hydrophobicity and describe their relationships with water molecules.
- Explain why certain liquids do not mix, based on concepts of miscibility, hydrogen bonds and hydrophobicity.
- Relate the physical properties of water and hydrophobic compounds to groundwater contamination and remediation.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to predict and/or describe phenomena. Alignment agreement: | Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. Alignment agreement: Solids may be formed from molecules, or they may be extended structures with repeating subunitsAlignment agreement: | Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Missouri - Science
-
Science and technology affect, and are affected by, society
(Grades
K -
5)
More Details
Do you agree with this alignment?
-
Human activity is dependent upon and affects Earth's resources and systems
(Grades
6 -
7)
More Details
Do you agree with this alignment?
-
Science understanding is developed through the use of science process skills, scientific knowledge, scientific investigation, reasoning, and critical think
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/wst_environmental_lesson03] to print or download.Pre-Req Knowledge
Have students complete the two associated activities before conducting this lesson.
Introduction/Motivation
(To best engage the students, begin this lesson AFTER they have completed the two associated activities(Refer to the activities: Density Column Lab - Part 1 and Density Column Lab - Part 2); this way, they have tangible experience with the behavior of these liquids before learning more about the theoretical concepts. As visual aids during the Introduction/Motivation and while presenting the Lesson Background information, have ready a beaker, water, and some type of alcohol [such as ethanol, isopropanol or methanol] dyed with food coloring. Optionally, have ready an oil/water density column as prepared for the Part 2 activity, and liquid laundry or dish detergent, no more than a cup.)
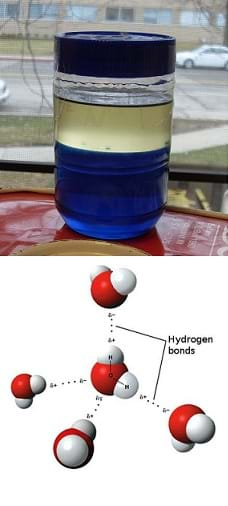
Now that we have conducted the density column lab activities, why do you think the layers don't mix together? (Listen to student ideas. Expect students to suggest that the separation is caused by the different liquid densities.) The different densities of the liquids explain where they align in the density column, but do not explain why none of the layers mixed.
(Pass around a beaker containing a solution of water mixed with food coloring-dyed alcohol so students can see that in this case the layers mix, even though the density of alcohol is less than the density of water.) Take a look at this example. In this beaker, the density of the alcohol is less than that of water, but the layers mix. This mixing of alcohol and water is similar to many cases of contaminated groundwater. It is common that contaminants mix with water in our drinking water sources and we are unable to know this by looking at the water.
Let's learn some new terms. (Write two words on the board: miscible, immiscible.) What is miscible? Miscible means capable of mixing without separating into two phases. What is immiscible? Immiscible means incapable of mixing. (Show them the oil/water density column.) Are these three liquids miscible or immiscible? (Answer: Immiscible.)
What do you think causes some contaminants to mix well with water and others, such as oil from oil spills, to not mix with water at all? (Listen to student ideas.) To answer this question, let's learn a little more about how liquids interact.
(Continue on to present students with the content provided in the Lesson Background section, adjusting as needed, depending on how much chemistry knowledge students have.)
Lesson Background and Concepts for Teachers
Hydrogen Bonds
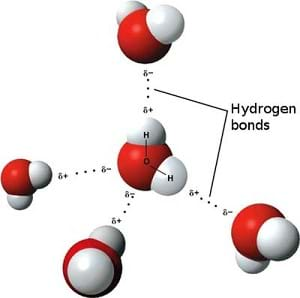
Why are the liquids in our oil/water density column immiscible? The answer lies in the chemical structure of water. What is the chemical structure of water? (Expect students to say H2O.) A water molecule can be written as H2O, but what we know as water is composed of more than one H2O molecule. So how could we expand on our understanding of the structure of water? How might we draw it?
(Draw a diagram using the form in Figure 1 or project the image.) Here we have added information about the charge distribution and how the hydrogen atoms from other water molecules are attracted to the negatively charged oxygen atom. Because oxygen has a greater electronegativity than hydrogen, meaning it has a greater tendency to attract electrons, the shared electrons between the hydrogen and oxygen bonds spend more time around the oxygen atom. Because of this, the oxygen atoms have slightly negative charges and the hydrogen atoms have slightly positive charges.
Also notice that hydrogen has only one electron while oxygen has six electrons for forming bonds. Since two hydrogen bonds are formed (one electron from each atom forms a bond), two oxygen electrons have no atoms to share. Therefore, other positively charged hydrogen atoms from neighboring water molecules are drawn to the free electrons, just as the negatively charged electrons are drawn to the positive charge of the nearby hydrogen atoms. This situation forms the basis of the hydrogen bond; it is actually just a force (based on the charges), but the force is so strong that we call it a bond. It is the existence of free electrons on the oxygen atoms that allow for hydrogen bonding, and these hydrogen bonds between water are what help it to be so stable.
Hydrophobicity
Let's learn some new terms. (Write two words on the board: hydrophobic, hydrophilic.) What is a phobia? (A fear of something.) What does hydro mean? (It means water.) Therefore, hydrophobic must mean "fear of water," or "water-hating." Likewise, hydrophilic means "water-loving." What makes molecules either hydrophobic or hydrophilic is their ability to form these hydrogen bonds. Hydrophilic molecules can form hydrogen bonds while hydrophobic compounds cannot.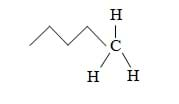
(Draw on the board a representation of an oil molecule, like Figure 2.) Here is a drawing that shows the chemical composition of an oil molecule (C5H12, pentane). The zigzag line represents the existence of more carbon-hydrogen bonds. Unlike oxygen, this carbon atom has no free electrons since they are all shared by hydrogen atoms. Therefore, this molecule is not capable of forming hydrogen bonds—it must be hydrophobic. To sum it up, hydrophilic liquids can form hydrogen bonds and mix with water, while hydrophobic liquids cannot form hydrogen bonds or mix with water.
What happened when you added detergent to your density columns? (Listen to student descriptions from what they observed at the end of the Density Column Lab – Part 2 activity, or [if available] add liquid laundry or dish detergent to the teacher's demonstration oil/water density column as an example for students to observe. Add enough to fill the density column container, being careful not to overfill. This causes the oil and water layers to mix, without any stirring.) All detergents and soaps have both hydrophobic and hydrophilic parts, which permits water to interact with oil and dirt (which is hydrophobic) to better clean our dirty clothes, dishes, etc.
Associated Activities
- Density Column Lab - Part 1 - Prior to conducting the Density & Miscibility lesson, have students complete this activity in which they calculate and compare the densities of four irregularly shaped solid objects through measurements conducted with triple beam balances and graduated cylinders (density = mass per unit volume). They also learn that environmental engineers focusing on water remediation must understand physical characteristics (such as density) of contaminants in water sources.
- Density Column Lab - Part 2 - Students calculate the densities of three common household liquids and compare them to the densities of each other and the four irregularly shaped solid objects examined in the Density Column Lab - Part 1. The items are combined into "density columns," before adding detergent and observing what happens. This conclusion is a perfect lead-in into the Density & Miscibility lesson, to learn why certain liquids mix, while others do not.
Lesson Closure
With what you now know about how liquids interact, who can explain what you saw in the density columns we created? Why does oil float on top of the water and why does it not mix with water? Can you explain it to me? (Listen to student answers and correct as necessary. Answer: The oil floats above water because it is less dense than water; it does not mix with water because it is hydrophobic and is not capable of forming hydrogen bonds with water.)
What happened when we added detergent to an oil/water density column? (Listen to student explanations and correct as necessary. Answer: Without any stirring, the detergent caused layers to mix that wouldn't mix otherwise, such as oil and water. Detergents and soaps have both hydrophobic and hydrophilic parts, so they allow water to interact with oil and dirt [which is hydrophobic] for improved cleaning.)
In the world of environmental engineering, how might this relate to oil spills or other contaminants that enter our water supplies? (Many strategies for cleaning up oil spills rely on the properties of miscibility and immiscibility. One strategy is to collect oil using engineered devices called booms—temporary floating barriers. This approach takes advantage of the immiscibility of water and oil to successfully sequester the oil and separate it from the body of water. On the other hand, another strategy is to add chemicals called dispersants, which essentially act like the detergent in our density columns. These dispersants help to break down the oil into oil droplets and reduce surface tension between the oil droplets and water, allowing them to mix. Once they are able to mix, or become miscible, the oil is able to disperse through natural osmatic processes. With this approach, the goal is for the oil concentration to become diluted enough that it no longer endangers the natural environment of the body of water.)
Vocabulary/Definitions
electronegativity: The tendency of an atom to attract electrons.
hydrophilic: "Water-loving." Hydrophilic liquids can form hydrogen bonds and mix with water.
hydrophobic: Fear of water or "water-hating." Hydrophobic liquids cannot form hydrogen bonds or mix with water.
immiscible: Incapable of mixing.
miscible: Capable of mixing without separating into two phases.
Assessment
Building on What We've Learned: Begin class by asking students to recap what happened during the Density Column Lab activities. What materials did you use? What steps did you follow? What did you observe? Did your results match your predictions?
Post-Lesson Quiz: After presenting the lesson and concluding with the points made in the Lesson Closure section, have students complete the Density & Miscibility Quiz to assess how well they comprehended the new content presented. Students define vocabulary words, draw water molecules and label charges, identify which liquids mix together, and answer questions about real-world applications.
Lesson Extension Activities
Assign students to investigate and report back to the class on real-world water remediation efforts that cleaned up water supplies by taking advantage of the properties of water and contaminants.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students explore the chemical identities of polymeric materials frequently used in their everyday lives. They learn how chemical composition affects the physical properties of the materials that they encounter and use frequently, as well as how cross-linking affects the properties of polymeric mater...
Copyright
© 2013 by Regents of the University of Colorado; original © 2010 Washington University in St. LouisContributors
Jessica Ray; Phyllis Balcerzak; Barry WilliamsSupporting Program
GK-12 Program, School of Engineering and Applied Science, Washington University in St. LouisAcknowledgements
This curriculum was developed with support from National Science Foundation GK-12 grant number DGE 0538541. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: May 31, 2019




User Comments & Tips