Summary
In the presence of water, citric acid and sodium bicarbonate (aka baking soda) react to form sodium citrate, water, and carbon dioxide. Students investigate this endothermic reaction. They test a stoichiometric version of the reaction followed by testing various perturbations on the stoichiometric version in which each reactant (citric acid, sodium bicarbonate and water) is strategically doubled or halved to create a matrix of the effect on the reaction. By analyzing the test matrix data, they determine the optimum quantities to use in their own production companies to minimize material cost and maximize carbon dioxide production. They use their test data to "scale-up" the system from a quart-sized ziplock bag to a reaction tank equal to the volume of their classroom. They collect data on reaction temperature and carbon dioxide production. More advanced students are challenged to theoretically predict the results using stoichiometry.
Engineering Connection
The primary relationship to engineering featured in this activity is a demonstration of the relationships between bench scale and full scale. While the end goal may be to design a 50,000 gallon tank, the best path of discovery is to work with a smaller scale. The advantages of the smaller scale are that they 1) are less expensive and easier to operate and build, and 2) can be safer if anything unexpected happens because the surrounding environment can be controlled. The advantage of the large scale is that it directly reflects the true system, so no other testing is required in order to predict behavior.
The second engineering idea being illustrated through this activity is the engineer's responsibility to design systems that provide the maximum product with the least cost while remaining safe. For example, in this activity the reaction is endothermic and produces a gas. A poorly designed and sized tank has the potential to cause damage to people and property if the temperature decreases to a point at which the material fails. Additionally, if the tank is sized too small for the quantity of gas being produced, a tank explosion could cause catastrophic damage.
Learning Objectives
After this activity, students should be able to:
- Follow procedures to collect data while changing more than one variable.
- Create line graphs to represent the data.
- Interpret data to predict overall behavior of multiple variables.
- Describe how this activity applies engineering design principles, including cost analysis.
Goals:
- Math competency: Skills and appreciation for data collection and analysis used in real life.
- Quantitative literacy: Ability to follow procedures and provide recommendations.
- Engineering applications: engineering design process to control products of a chemical reaction, cost considerations corresponding to performance.
- Cultural relevancy: How to follow procedures, collaborative work in small groups and hands-on activities.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics, as well as possible social, cultural, and environmental impacts. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Evaluate a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | When evaluating solutions it is important to take into account a range of constraints including cost, safety, reliability and aesthetics and to consider social, cultural and environmental impacts. Alignment agreement: | New technologies can have deep impacts on society and the environment, including some that were not anticipated. Analysis of costs and benefits is a critical aspect of decisions about technology. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-6. Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: In many situations, a dynamic and condition-dependent balance between a reaction and the reverse reaction determines the numbers of all types of molecules present.Alignment agreement: Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed.Alignment agreement: | Much of science deals with constructing explanations of how things change and how they remain stable. Alignment agreement: |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Conduct research to inform intentional inventions and innovations that address specific needs and wants.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Evaluate ways that technology can impact individuals, society, and the environment.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Washington - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Washington - Science
-
Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics, as well as possible social, cultural, and environmental impacts.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 8 g citric acid (C6H8O7)
- 10.4 g sodium bicarbonate (baking soda)
- 40 ml water
- 2 quart-size ziplock bags
- thermometer (approximate range: 0-200 °F)
- syringe (approximate size 4 ml, for taking gas sample)
- tape measure
- calculator
- colored tape to seal syringe
- safety glasses, pair per student
- Exposed Reaction Worksheet
To share with the entire class:
- stopwatch
- (optional) use of GC for data analysis of CO2 production; the production of CO2 can also be estimated through calculations using the stoichiometric balance of the reaction equation
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/wsu_big_chill_activity1] to print or download.Pre-Req Knowledge
Students should be familiar with:
- Math concepts: volumes, graphing data, measuring and unit conversion, and conservation of matter.
- Science concepts: mass, temperature measurements.
Alternatively, this activity could be used as a means of introducing these concepts. It is also an ideal opportunity to introduce the subject material of: reactions, stoichiometry, endothermic vs. exothermic reactions, mass and mole conversions, and the ideal gas law.
Introduction/Motivation
In the presence of water, citric acid [C6H8O7] and sodium bicarbonate [NaHCO3] (aka baking soda) react to form sodium citrate [Na3C6H5O7], water, and carbon dioxide [CO2].
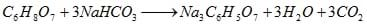
To keep the workers safe, chemical engineers must control the reaction temperature and pressure so no explosions occur. So the manufacturing company makes a profit, engineers must produce the most carbon dioxide [CO2] using the least citric acid [C6H8O7].
In groups of two, your engineering challenge is to test the reaction in the lab before a large-scale operation plant is turned on. To keep the plant employees safe and so the plant earns a profit, you must determine:
- The temperature change that occurs during the reaction for various ratios of reactants.
- The quantity of CO2 produced from the reaction using the same reactant ratios.
Procedure
Background and Concepts for Teachers
- Discuss the chemicals that will be used in the reaction. Have students discuss the physical characteristics of each.
- Sodium bicarbonate (aka baking soda). Students may be familiar with baking soda from household baking and cleaning. Baking soda works as an antacid, but would taste very bad alone. Sodium bicarbonate is also used to fight fires because at high temperature it turns to carbon dioxide and can smother fires.
- Citric acid is a common ingredient in citrus-flavored foods. Have students look for it in the ingredient lists for candy and soda beverages.
- The reaction of sodium bicarbonate and water is very similar to the phenomena seen when Alka-Seltzer tablets are placed in a glass of water.
- Discuss what a reaction is and how one might observe that a reaction is occurring.
- Demonstrate mixing citric acid + sodium bicarbonate. (Is a reaction occurring?)
- Demonstrate adding water to the mixture? (Is a reaction occurring?)
- Relevance: acid + base = water + gas + salt (the product is neutral). Example applications: 1) Ant-acids neutralize stomach acid. 2) Bases used for reclaiming land used during mining (the water is very acidic and needs to be treated to be neutralized). 3) Acids and bases used to promote plant growth. Neutralizing acidic or basic soil to promote plant growth.
- Discuss the connections to engineering.
- Safety aspect: over pressure or extreme temperature can cause catastrophic failure.
- Profit aspect: using less citric acid to produce more carbon dioxide. (Is it possible?)
- Overview of the experimental plan over the three days.
Specific Procedure
- Background: It is recommended to divide the class into four groups so each "engineering team" performs a different reactant ratio on Day 2. This way, the entire matrix can be tested without all students needing to perform five sets of experiments. Suggested tests:
On Day 1: Everyone: 2.6 g citric acid + 2.6 g sodium bicarbonate
On Day 2:
- Group A: 1g citric acid + 2.6 g sodium bicarbonate
- Group B: 4 g citric acid + 5.2 g sodium bicarbonate
- Group C: 2 g citric acid + 5.2 g sodium bicarbonate
- Group D: 6 g citric acid + 7.8 g sodium bicarbonate
- Before the Activity
- Collect the required reactants.
- Gather the remaining items on the Materials List.
- Make copies of the Exposed Reaction Worksheet, one per team, which includes the procedures.
- On the classroom board or overhead projector, prepare a large data table for all students to record their findings (from Day 2). Make it visible for the entire class and suitable to serve as a visual aid in the discussions at the end of Day 2.
- With the Students
Day 1: Experimental Methods
- Present the introduction content, as described above.
- Divide the class into groups of two students each.
- Review the test procedures and how to use the given materials.
Day 2: Data Collection
- Student groups conduct the same reaction using different assigned quantities of reactants.
- Students collect, label and seal gas samples.
Day 3: Data Analysis, Discussion & Scale-up
- Provide gas analysis results.
- Calculate cost of reactants vs. profit from products.
- Scale-up activity.
Vocabulary/Definitions
products: All the materials formed through a reaction. Generally "what we end up with."
reactant: Starting materials for a reaction. Generally elementally rearranged during the reaction.
Assessment
Review students' completed Exposed Reaction Worksheets to gauge their depth of comprehension.
Optional post-activity assessment: student reflection by class discussion or written assignment.
Safety Issues
- Remind students not to drink any chemicals, even those labeled water or soda, because contamination is always possible, especially in chemical laboratory settings.
- Have students wear safety goggles at all times.
Activity Scaling
For more advanced students, challenge them to first calculate how much CO2 should be produced theoretically in each reaction using stoichiometry, before actually doing the experiment. As a bonus question, ask students: Is there any way to calculate theoretically how much carbonic acid should be produced in each experiment? At the end of the experiment, have students compare their theoretical to actual answers. Ask them to explain why their experimental results will probably never be the same as theoretical results. (Possible answers: Perhaps not all CO2 produced will be collected; perhaps not all of the limiting reactant will react to produce the theoretical product quantities.) Refer to the Theoretical Results Using Stoichiometry Solution Guide .
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Your students have been hired to build a pop rocket, but on a tight budget. Engineering design usually has some constraints and you won’t always have access to the materials you think you might need. But through brainstorming and trial and error, a viable rocket launch is definitely possible!

Students learn how rocket thrust is generated with propellant. The two types of propellants are discussed—liquid and solid—and their relation to their use on rockets is investigated. Students learn why engineers need to know the different properties of propellants.
Copyright
© 2013 by Regents of the University of Colorado; original © 2009 Board of Regents, Washington State UniversityContributors
Courtney BonuccelliSupporting Program
CREAM GK-12 Program, Engineering Education Research Center, College of Engineering and Architecture, Washington State UniversityAcknowledgements
This content was developed by the Culturally Relevant Engineering Application in Mathematics (CREAM) Program in the Engineering Education Research Center, College of Engineering and Architecture at Washington State University under National Science Foundation GK-12 grant no. DGE 0538652. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: July 27, 2023





User Comments & Tips