
Summary
Rockets need a lot of thrust to get into space. Students learn how rocket thrust is generated with propellant. The two types of propellants are discussed—liquid and solid—and their relation to their use on rockets is investigated. Students learn why engineers need to know the different properties of propellants by drawing real world examples from the associated activities.Engineering Connection
Engineers use powerful propellants to launch rockets into space. When these propellants burn, hot gas is expelled out the nozzle causing the rocket to move forward. To achieve the necessary speeds, engineers must understand the chemical properties of the different propellants. Factors such as weight, efficiency, controllability and safety must be kept in mind when choosing a propellant. Specific propellants are better for different tasks, and it is the job of engineers to choose which and how much of a propellant to use.
Learning Objectives
After this lesson, students should be able to:
- Apply Newton's third law of motion to explain how a rocket launches.
- Explain the difference between propellant and fuel.
- Describe how fuel weight affects the ability to get a rocket into space.
- Explain why engineers need to know the properties of different propellants.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Objects in contact exert forces on each other.
(Grade 3)
More Details
Do you agree with this alignment?
| NGSS Performance Expectation | ||
|---|---|---|
|
3-PS2-1. Plan and conduct an investigation to provide evidence of the effects of balanced and unbalanced forces on the motion of an object. (Grade 3) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation collaboratively to produce data to serve as the basis for evidence, using fair tests in which variables are controlled and the number of trials considered. Alignment agreement: Science investigations use a variety of methods, tools, and techniques.Alignment agreement: | Each force acts on one particular object and has both strength and a direction. An object at rest typically has multiple forces acting on it, but they add to give zero net force on the object. Forces that do not sum to zero can cause changes in the object's speed or direction of motion. (Boundary: Qualitative and conceptual, but not quantitative addition of forces are used at this level.) Alignment agreement: Objects in contact exert forces on each other.Alignment agreement: | Cause and effect relationships are routinely identified. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
3-PS2-2. Make observations and/or measurements of an object's motion to provide evidence that a pattern can be used to predict future motion. (Grade 3) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Make observations and/or measurements to produce data to serve as the basis for evidence for an explanation of a phenomenon or test a design solution. Alignment agreement: Science findings are based on recognizing patterns.Alignment agreement: | The patterns of an object's motion in various situations can be observed and measured; when that past motion exhibits a regular pattern, future motion can be predicted from it. (Boundary: Technical terms, such as magnitude, velocity, momentum, and vector quantity, are not introduced at this level, but the concept that some quantities need both size and direction to be described is developed.) Alignment agreement: | Patterns of change can be used to make predictions. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
A transportation system may lose efficiency or fail if one part is missing or malfunctioning or if a subsystem is not working.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Develop a scientific explanation regarding relationships of the components of the solar system
(Grade
4)
More Details
Do you agree with this alignment?
Pre-Req Knowledge
A familiarity with Newton's three laws of motion, as presented in the previous three lessons of this unit.
Introduction/Motivation
Have you ever seen a real rocket being launched? If you have, I bet you noticed a lot of fire and smoke. Why was that happening? Imagine how hot it would be if you were standing close to the launch pad! Do you remember how a rocket moves? (Refer to Lesson 2: Newton Gets Me Moving.) That's right, the rocket moves by shooting mass (hot gas) from the vehicle at a high velocity or speed. Whose law was this? (Answer: Newton's third law of motion) What does Newton's third law say? Remember, it states that for every action there is an equal and opposite reaction. So, when the rocket shoots mass (hot gas) out in one direction, the rocket actually moves away in the opposite direction! The direction it moves in is a result of thrust.
Launching a rocket into space requires a lot of energy. That energy comes from something called propellant. Did you know that a car uses propellant? A car burns gasoline and air in its engine so that the car can travel down the road. Rockets and cars are similar in this way, but a rocket has to travel 62 miles above the Earth to reach space at a speed of 25,000 miles per hour. How does it do it?
In order to design Tess' rocket, her engineering team (that's you!) needs to understand what sort of propellant to use, how propellants work and finally, how much to use. Propellant is a little bit different from fuel in that propellant contains both fuel and oxidizer.
Let's start with the oxidizer. What chemical does the word oxidizer resemble? (Answer: Oxygen.) What does fire needs in order to burn? (Demonstration: Light a candle in front of the class and ask: what are the ways that we could put the flame out? Place a larger glass on top of the candle cutting off its air supply. Expect students to realize that the fire needs something in the air in order to burn. Oxygen!.) That is correct: fire needs oxygen to burn. What does oxidizer mean, then? It is a substance that supplies oxygen in order to burn a fuel.
So now that we know about an oxidizer, let's learn about the propellant? Propellant is generally what we think of as fuel. In the case of the candle, what is the fuel? (Answer: Wax.) What types of fuel can you list? (Possible answers: gasoline, natural gas, propane, kerosene, wood, coal, wax, etc. For help in prompting some answers, ask students about appliances and vehicles in their houses and how they think they run.) These are all examples of fuels. Which ones are examples of liquid fuel? (Possible answers: gasoline, kerosene, etc.) Which ones are examples of solid fuel? (Possible answers: wood, coal, wax, etc.)
Choosing an appropriate fuel for Tess' rocket is very important. What factors affect our choice? (Answer: Weight and rate of burning are perhaps the most important factors.) Weight is incredibly important because fuel weighs a lot. And, we have to launch the rocket with all the fuel attached to it. Additionally, we have to have enough fuel to get Tess, the rocket and the satellites all the way into space!
Ideally, any rocket—including Tess'—is approximately 90% propellants, 4% tanks, engines, fins, etc. and 6% payload (spacecraft, satellites and astronauts). That is a lot of propellants! Basically, that means that the weight of the propellant, the tanks and the payload must add up to 100(%). Let's try a short mental math exercise. If Tess' satellite weighs 6 tons, and the rocket's tanks, engines and body weigh 4 tons, how many tons would the propellants weigh? (Answer: 6 + 4 =10. Then, 100 – 10 = 90. The answer is 90 tons. The total of all of them is 100 tons). How about another one: if the total rocket weighs 100 pounds (it's a small rocket), how much of that weight should be propellant? (Answer: 90% of 100 pounds is 90 pounds—because we already know that 90% of the total weight is propellant.) How about the payload and tanks together? (Answer: 100 pounds – 90 pounds = 10 pounds. Together, the payload and tanks should weigh 10 pounds.)
Now that we have a basic concept of propellants and their importance in getting Tess and her cargo into space, we can learn more about how propellants work and how rocket thrust is generated. Are you ready, engineers? We're ready for launch!
Lesson Background and Concepts for Teachers
Rockets are able to travel into space because of a force called thrust. How is thrust generated? The answer is propellant. When rocket propellant is burned, hot gases expand very quickly through a nozzle. These hot gases are forced out the back of the rocket, which produces an equal and opposite direction upward, as described by Newton's third law of motion.
What exactly is propellant and how does it work? Propellant is made of a fuel and an oxidizer. Two types of propellant are used in chemical rockets: liquid and solid.
Liquid Propellant

Liquid propellant is composed of liquid fuel and oxidizer. Rockets use many different combinations of these two substances. One example of liquid propellant is liquid hydrogen with liquid oxygen. The large, orange external fuel tank on NASA's space shuttle holds both of these two chemicals (see Figure 2).
You cannot see it from the outside, but the chemicals are separated from one another until launch time. Then during the launch procedure, the chemicals flow through pipes and valves until they reach a chamber where they are burned. The burning of this propellant creates a high pressure and high velocity gas. This gas travels through an exit nozzle that makes the gas move even faster. The faster the gas leaves the nozzle, the more thrust the rocket has.
The liquid propellant on the space shuttle is used to fire the three main engines on the orbiter. These engines move in a specified order to control the direction of the shuttle. Liquid propellant is good to use because it can provide a lot of thrust. Also, rockets using liquid propellant can be quickly shut down if a problem arises—an extremely important safety feature. However, because of how complex they are, these types of chemical rockets can be very expensive to design and build. This is why other rockets use a different type of propellant, called solid propellant.
Solid Propellant
Many people do not think of fuel as being solid. However, many ordinary examples of solid fuel are in every day use, such as wood and coal. Even candle wax is a solid fuel. Energy is slowly released as the wax melts and is consumed by the flame. Solid propellant used in rockets utilizes substances that are a bit more complex and burn at a much faster rate, but the idea is still the same. Refer to the Fuel Mystery Dis-Solved! activity to investigate what variables affect the rate of reaction of solid propellants. The most famous solid rockets are the two white solid rocket boosters (SRBs) that are attached to the orange external tank on the space shuttle (remember, the orange external tank uses liquid propellant. The solid fuel used in the SRBs is atomized aluminum, and the oxidizer is ammonium perchlorate.
Solid propellant rockets may have a hole drilled through the center that is shaped like a circle or star (or other more complicated shape). The interior shape of the hollowed out core is an important factor in determining a rocket's performance. The amount of propellant exposed to burning flames is called surface area. An increase in surface area increases thrust but reduces burn-time since the propellant is being consumed faster. Typically, solid rockets start out having a large propellant surface area because rockets need the greatest amount of thrust to get off the ground. After the propellant has burned for some time, the surface area decreases since the required thrust is less. For example, a star configuration accomplishes this necessary surface area change over time in that as the propellant burns, the star eventually becomes circular and that surface area is less. The SRBs on the space shuttle use an 11-point star configuration.
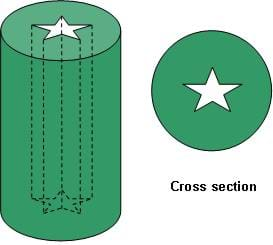
Solid propellant rockets are relatively simple. Unfortunately, once a solid rocket is ignited, it consumes all of its propellant without any option to turn it off. So, if anything goes wrong, this approach can potentially pose a dangerous situation. Because of this, the SRBs are the last component to be turned on before launch so that if anything goes wrong before then, the mission can be aborted.
Thrust for a rocket continues as long as its engines have propellant that can be burned. Furthermore, the mass of the rocket changes during flight. Its mass is the sum of all its parts, including engines, fuel tanks, payload, control system and propellant. Students can investigate how solid fuels produce thrust with the hands-on design activity, Aqua-Thrusters! By far, the largest part of the rocket's mass is its propellant (note: earlier in this lesson, we learned that the propellant accounts for 90% of the weight of the rocket). This mass constantly changes as the engines burn and eject the propellant—ultimately decreasing the rocket's mass during flight. The acceleration of the rocket increases as its mass decreases, as described by Newton's second law of motion, which states that the force of an object is equal to its mass times its acceleration. A rocket is accelerating fastest when its propellant is almost gone. Newton's second law of motion can be restated as such when explaining rockets: the greater the mass of propellant ejected, combined with the speed with which is it is ejected from the engine, then the greater the thrust force which pushes the rocket in the opposite direction.
Simulating Rocket Launches
Did you know that fireworks that shoot up into the air are rockets? Gunpowder is often used in fireworks as a solid propellant. You could launch a model rocket into the air with gunpowder to simulate a real rocket launch. A safer alternative, however, is to use the energy stored in an antacid tablet (such as Alka-Seltzer®), which contains aspirin, sodium bicarbonate (NaHCO3) and citric acid (C6H8O7). When the tablet is dissolved in water (or some other liquid), a chemical reaction occurs between the sodium bicarbonate and the citric acid: the hydrogen ions (H+) and carbonate ions (CO6 -2) are freed to collide and react in the solution. The products of the reaction are carbon dioxide (CO2) gas and water. The carbon dioxide can be seen as bubbles in the gas. If you find a way to capture the carbon dioxide created, you can release this pressure and use it to create thrust. Students can put their new knowledge to the test by designing their own rocket with the fun and hands-on Pop Rockets activity.
Associated Activities
- Fuel Mystery Dis-Solved! - Students discover how solid fuels are consumed and what variables affect the rate of reaction.
- Aqua-Thrusters! - Students investigate how solid fuels produce thrust by constructing rocket-powered boats.
- Pop Rockets - Students launch paper rockets using antacid tablets as solid fuel.
Lesson Closure
In this lesson, we learned that rockets can be launched into space by expelling propellant in the opposite direction. Which law describes this? That's right, Newton's third law of motion! Newton's second law tells us that force equals mass times acceleration. So, we know that the larger the amount of propellant and the faster it is pushed out the back of the rocket, the more thrust the rocket has. Also, we learned that rockets use either liquid or solid propellants. Propellants are made of a fuel and an oxidizer. What is an oxidizer? (Answer: Something that provides oxygen to be burned.) Different fuels are able to provide different amounts of energy at different rates, and it is the job of engineers to find fuels that provide the most energy for the rocket. This means that engineers must also think about the weight and cost of the fuel.
In order for Tess to be able to explore space and to carry the satellites that will be part of the rocket cargo for use to communicate with her mother, Maya, her rocket needs to successfully make it into space. Since you, as the engineering team, now understand propellants and how they work, you know one more important factor necessary to design Tess' rocket.
Vocabulary/Definitions
chemical reaction: A process in which one type of substance is chemically converted to another substance involving an exchange of energy.
fuel: Something consumed to produce energy.
gas: A collection of molecules with enough energy to remain isolated and free floating (as opposed to liquids and solids where particles group together).
oxidizer: A substance that supplies oxygen in order to burn a fuel.
pressure: Force per area. Pressure results from collisions of gas molecules with a surface. Measured in units of lb/in2 (psi) or N/m2 (Pa).
surface area: The amount of exposed or outer material of any object.
thrust: The forward-directed force of a jet or rocket engine as a reaction to the ejection of exhaust gases.
Assessment
Pre-Lesson Assessment
Discussion Question: Solicit, integrate and summarize student responses.
- Ask students if candy lasts longer when you chew it or suck on it. (Answer: Sucking lasts longer. If you chew it into many pieces you increase the candy's surface area and your saliva will dissolve it faster!) Do you get more candy if you chew it up? (Answer: No, it is the same amount of candy. It just does not last as long.) These are important concepts to understand for learning about solid fuel.
- Shake up a can of soda. Open the can over a sink or outdoors. Ask students what happened to cause the pop to explode. (Answer: The liquid is carbonated with carbon dioxide gas. The gas is usually dissolved into the liquid because the can is pressurized. When you shake the can, some of the gas is no longer dissolved and bubbles form on the sides and bottom of the can. When you open the can, the undissolved gas bubbles try to escape and push liquid out in the process since it is in the way.)
Post-Introduction Assessment
Question/Answer: Ask students the following questions and have them raise their hands to respond. Write their answers on the classroom board.
- Does launching a rocket into space require energy? (Answer: Yes.)
- What material is used to generate thrust in a rocket? (Answer: Propellant.)
- What are the two types of propellant? (Answer: Liquid and solid.)
- What is the difference between a propellant and fuel? (Answer: Propellants are made of a fuel and an oxidizer.)
- What is used as propellant in cars? (Answer: Gasoline and air; gasoline is the fuel and air is the oxidizer.)
- Why do engineers need to know the properties of different propellants? (Answer: Engineers must know about the properties of propellants in order to design rockets that safely and successfully launch into space.)
Class Discussion: Show pictures or video clips of rocket launches (from NASA's website at http://search.nasa.gov/nasasearch/search/search.jsp?nasaInclude=launch+video+clips or from v2Rocket.com at http://www.v2rocket.com/start/others/aud_vid.html). In groups of 2-3, have students discuss what powers rockets. (Expect fire and fuel as popular answers.) Fire is the result of fuel burning, but how exactly does the fuel store and release energy? (Answer: Fuel stores chemical energy that is released when the fuel burns, causing an expansion of gases and a pressure increase. These gases are expelled out of the back of the rocket very quickly through a nozzle, which makes the rocket move in the opposite direction, as described in Newton's third law of motion).
Lesson Summary Assessment
Mental Math/Pairs Check: Have students work in pairs to answer the following questions.
Ideally, any rocket, is approximately 90% propellants; 4% tanks, engines, fins, etc.; and 6% payload (spacecraft, satellites and astronauts). Think of a pie graph when picturing the distribution; the biggest portion is propellants.
- If a satellite weighs 5 tons, and the tanks, engines, and body of the rocket weigh 5 tons, how many tons would the propellants weigh? (Answer: 5 + 5 = 10 tons. 100 – 10 = 90 tons. The answer is 90 tons.)
- Challenge: If the total rocket weighs 200 tons, how much of that weight should be propellant? (Answer: 90% of 100 tons = 90 tons, so 90% of 200 tons = 180 tons. The answer is 180 tons.)
Toss-a-Question: Ask students to independently think of an answer to each of the following questions and write it on a half sheet of paper. Have students wad up and toss the papers to another team member who then adds his/her answer. After all students have written down ideas, have them toss the paper wads to another student, who then reads the answers aloud to the class. Discuss the answers as the class.
- Can any material be fuel? (Answer: No, only materials that react with something and cause a change in energy of its particles can be fuel.)
- How is pressure created in a rocket engine? (Answer: A reaction takes place that gives particles more energy causing them to move more and take up more space which increases the pressure.)
- How does the amount of surface area affect the rate at which fuel burns? (Hint: Remind students to think about the candy chewing example from the pre-assessment discussion.) (Answer: More surface area means a faster reaction; less surface area results in a slower burn.)
Lesson Extension Activities
Show students a rocket launch by going to NASA's website and downloading a video clip of any shuttle launch! http://www.nasa.gov/
Class Debate: Working in groups of four, have two students argue in favor of solid propellant on the latest NASA rocket to Mars and the other two argue in favor of liquid propellant. Have students report back to the entire class on how their debates went and if they came to any agreements.
Have students investigate other methods of propulsion instead of chemical rockets, such as ion and nuclear rockets.
Assign students to research the differences between jet engines and chemical rocket engines.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

The purpose of this lesson is to teach students how a spacecraft gets from the surface of the Earth to Mars. Students first investigate rockets and how they are able to get us into space. Finally, the nature of an orbit is discussed as well as how orbits enable us to get from planet to planet — spec...

Students acquire a basic understanding of the science and engineering of space travel as well as a brief history of space exploration. They learn about the scientists and engineers who made space travel possible and briefly examine some famous space missions.

Students explore motion, rockets and rocket motion while assisting Spacewoman Tess, Spaceman Rohan and Maya in their explorations. First they learn some basic facts about vehicles, rockets and why we use them. Then, they discover that the motion of all objects—including the flight of a rocket and mo...

Through the continuing storyline of the Rockets unit, this lesson looks more closely at Spaceman Rohan, Spacewoman Tess, their daughter Maya, and their challenges with getting to space, setting up satellites, and exploring uncharted waters via a canoe. Students are introduced to the ideas of thrust,...
References
Armstrong, Dennis. "Space Shuttle Overview: Discovery (OV-103)," NASA Orbiter Fleet. September 15, 2005. National Aeronautics and Space Administration. Accessed 2005. http://www.nasa.gov/centers/kennedy/shuttleoperations/orbiters/discovery-info.html
Benson, Tom. Beginner's Guide to Model Rockets. National Aeronautics and Space Administration. Accessed 2005. http://www.grc.nasa.gov/WWW/K-12/rocket/bgmr.html
Speake, Vicki. Science is Here: E-SET, "What Puts the Fizz in Alka-Seltzer," September 2002. Iowa State University, University Extension. Accessed 2005. http://www.extension.iastate.edu/e-set/science_is_here/alkaseltzer.html
Copyright
© 2005 by Regents of the University of ColoradoContributors
Jeff White; Brian Argrow; Luke Simmons; Jay Shah; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the DOE or NSF, and you should not assume endorsement by the federal government.
Last modified: September 28, 2019







User Comments & Tips