Quick Look
Grade Level: 4 (3-5)
Time Required: 45 minutes
Expendable Cost/Group: US $1.00
Group Size: 2
Activity Dependency: None
Subject Areas: Earth and Space, Physical Science, Science and Technology
NGSS Performance Expectations:

| 5-PS1-4 |
Summary
Students investigate the simulated use of solid rocket fuel by using an antacid tablet. They observe the effect that surface area and temperature has on chemical reactions. They also compare the reaction time using two different reactants: water and vinegar. Finally, students report their results in bar graph format. In the continuing hypothetical scenario of this unit, what students learn builds their background knowledge towards designing the best rocket to get their cargo into space.
Engineering Connection
Engineers must understand chemical reactions when designing rocket propellants. Safety, amount of thrust and amount of heat are all important considerations for rocket performance. Engineers must know and understand the properties of fuel in order to design rockets that effectively maximize fuel consumption.
Learning Objectives
After this activity, students should be able to:
- Describe how temperature and surface area exposure affect the rate at which fuel is consumed.
- Explain why engineers want to know about the properties of a fuel when designing rockets.
- Create a bar graph of collected experimental data.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-PS1-4. Conduct an investigation to determine whether the mixing of two or more substances results in new substances. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Conduct an investigation collaboratively to produce data to serve as the basis for evidence, using fair tests in which variables are controlled and the number of trials considered. Alignment agreement: | When two or more different substances are mixed, a new substance with different properties may be formed. Alignment agreement: | Cause and effect relationships are routinely identified, tested, and used to explain change. Alignment agreement: |
Common Core State Standards - Math
-
Draw a scaled picture graph and a scaled bar graph to represent a data set with several categories. Solve one- and two-step "how many more" and "how many less" problems using information presented in scaled bar graphs.
(Grade
3)
More Details
Do you agree with this alignment?
-
Represent and interpret data.
(Grade
5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Energy comes in different forms.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Draw a scaled picture graph and a scaled bar graph to represent a data set with several categories.
(Grade
3)
More Details
Do you agree with this alignment?
-
Represent and interpret data.
(Grade
5)
More Details
Do you agree with this alignment?
Colorado - Science
-
Identify and describe the variety of energy sources
(Grade
4)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 3 solid halves and 3 crushed halves of antacid tablets, such as Alka-Seltzer™; do NOT use Tums™ tablets
- 1 clear plastic cup
- 1 stopwatch or clock with a second hand
- vinegar, enough to fill the clear plastic cup
- Fuel Analysis Worksheet, one per student; choose from two versions: for grades 2-3, for grades 4-5
- safety glasses/goggles, one pair per student
To share with the entire class:
- access to a sink to get and dispose of tap water
For a class demonstration:
- 1 tablespoon baking soda
- ¼ cup vinegar
- 1 clear cup or bowl
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_rockets_lesson04_activity1] to print or download.Pre-Req Knowledge
A basic understanding of Newton's three laws of motion is helpful.
Introduction/Motivation
Have you ever mixed baking soda with vinegar? What happens? (Mix the two ingredients in a clear bowl or cup so students can observe the reaction.) When these two substances are mixed together, they cause a chemical reaction and produce carbon dioxide. That is why you see a lot of fizzing and bubbling. A similar reaction occurs when you drop an antacid tablet in a cup of water. Pretty cool!
So, how does this relate to rockets? Some rockets use solid fuel propellant to generate thrust. What is thrust? Thrust is the forward motion of a rocket engine as a reaction to gas being ejected from it. That is how the rocket gets into space. Remember Newton's third law of motion? (The third law states that for every action there is an equal and opposite reaction.) The chemical reaction is set off in solid fuel rockets by lighting them on fire! Did we have to light the baking soda on fire to get it to work? No. Some of the factors that affect a chemical reaction are the same whether you have to light the fuel on fire or not. .
When rocket fuel is burning, a chemical reaction is occurring. Engineers who design rockets must understand this reaction. In order to get Tess and her cargo into space safely, it is very important to understand that chemical reactions are not only very useful, but if not done with full understanding, can also be potentially dangerous. How the different chemicals react affects how much thrust will be created, as well as how much pressure and heat will be created. The conditions under which the chemical reaction occurs also affect its outcomes. As Tess' engineering team, this information is critical to you when trying to decide how much propellant is needed, how strong the rocket must be, and how much heat shielding the rocket needs (burning fuel can be hot!). Today, we will perform an experiment that to see what affects chemical reactions. This will help us design the best rocket to get our cargo into space.
Procedure
Chemical Reaction Background
The chemical reaction that occurs in the body with an antacid tablet is as follows: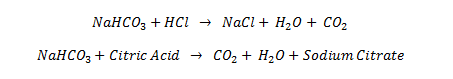
The sodium bicarbonate (NaHCO3) in the antacid tablet reacts with the hydrogen chloride (HCl) acid in the stomach to neutralize it, producing carbon dioxide (CO2) as a byproduct.
Essentially, what is happening in these reactions (with vinegar and water) is that a proton (H+) is being freed up by being in a liquid solution, as is HCO3 - from the sodium bicarbonate and is described by the following chemical equation: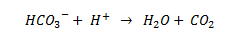
Before the Activity

- Gather materials and make copies of the Fuel Analysis Worksheet, choosing the one that is suitable for your students' grade level.
- Remove the antacid tablets from their packaging and break them in half. Prepare the crushed tablets and clean up any spilled white powder. Refer to Figure 1 and the Troubleshooting Tips section.
With the Students
- Divide the class into groups of two students each. Hand out the worksheets.
- Give each group one cup filled with cold tap water and a solid half tablet of antacid. (Note: Hand out antacid tablets only as needed so that you know that all tablets are accounted for and used appropriately in the experiment.)
- Have one student start the stopwatch as another student puts the half tablet of antacid into the cup of water.
- Have the student timers stop the stopwatches when the tablet is completely dissolved and they no longer hear/see the fizz.
- Direct the students to record their times and observations on the worksheets before continuing.
- Have students empty their cups into the sink and fill them back up with cold tap water—the same amount of water as they had before.
- Give each group a crushed half tablet of antacid.
- Repeat steps 3-5.
- Have students empty out their cups in the sink. This time, have them fill them up with hot water—to the same level that they previously filled their cups with cold water. Direct them to repeat the experiment for each of a solid half tablet and a crushed half tablet. Have them record their results on the worksheets.
- Have students empty their cups in the sink. This time, have them fill them with vinegar—to the same level as they filled with water. Direct them to repeat the experiment two times again, using each of a solid half tablet and a crushed half tablet. Have them record their results on the worksheets.
- Then have students complete the worksheet by making a bar graph of their results and answering the final question.
Assessment
Pre-Activity Assessment
Asking Questions: Before the activity, have students come up with questions about what they think will happen to a reaction given different scenarios (i.e., if the temperature of water changes). Guide them to ask questions about which will dissolve faster. If they need help, present these questions:
Of the scenarios listed below, have students predict which will dissolve faster. Record their predictions on the classroom board.
- Solid antacid tablet or crushed antacid tablet?
- Antacid in hot water or cold water?
- Antacid tablet in water or vinegar?
Activity Embedded Assessment
Worksheet/Pairs Check: Have students work individually or in pairs on the Fuel Analysis Worksheet. After students finish the worksheet, have them compare answers with a peer or another pair, giving all students time to finish the worksheet.
Post-Activity Assessment
Prediction Analysis: Have students compare their initial questions and predictions with their test results, as recorded on the worksheets. Ask the students to explain why they think the tablet dissolved faster when they are crushed or in hot water using their observations and data from the activity.
Numbered Heads: Have students on each team number off so that each member has a different number. Ask them a question (give them a time frame for solving it, if desired). Direct the members of each team to work together to answer the question. Everyone on the team must know the answer. Call a number at random. Students with that number raise their hands to give the answer. If students do not know the answers, give teams more time. Ask the students:
- Does a crushed antacid tablet dissolve faster than a solid tablet in a liquid? (Answer: Yes, crushed tablet dissolves faster.)
- Why does more surface area (crushed tablet) decreases the time it takes for the tablet to dissolve? (Answer: More surface area means more molecules of the antacid can react with the liquid molecules immediately.)
- How are antacid tablets related to solid rocket fuel? (Answer: Both contain stored energy. When a chemical reaction is started, this energy is released and can be used.)
- Does an antacid tablet dissolve faster in hot or cold water? (Answer: Hot; it has more energy to react with the antacid.)
- Why do you think hot water increases the rate of reaction? (Answer: Warm water moves faster than cold water. It has more energy in the form of water molecules that vibrate and move around faster than those in cold water. These faster moving warm water molecules find and react with antacid molecules much quicker than cold water molecules.)
- Should engineers design a dishwasher to use solid blocks of soap or powered soap if they want the soap to mix with the water quickly? (Answer: Powder, since it has more surface area.)
- Which cleans dishes better, hot or cold water? (Answer: Hot water because the extra energy of motion makes the hot water particles mix with the soap faster! Although unrelated to chemical reactions, the heat also softens the food making it easier to remove.)
Safety Issues
Remind students not to put the antacid tablets (crushed or solid) in their mouths; eating a full tablet could make a student very sick.
Make sure students wear safety glasses when putting the antacid into the water.
Hand out antacid tablets only as necessary; do not give each group a "supply" in advance.
Troubleshooting Tips
Recommended preparation method for solid and powder forms of half tablets:
- Use scissors to cut a two-tablet packet down the middle (between two tablets).
- Carefully tear open each of the foil antacid packets and remove both tablets. Break them in half as evenly as possible.
- Place half of each tablet back into its foil packet.
- Fold over the open end of one of the packets, hold it shut and use a blunt object to crush the half tablet in the packet (this takes some practice but works well). Repeat for the other tablet.
- Now you have two crushed half-tablets nicely contained in their packets and two solid half-tablets set aside.
Activity Extensions
Have students repeat the experiment at a number of different liquid temperatures. Have them record the temperature with a thermometer and make a new graph of the results.
Have students repeat the experiment with antacid tablets cut into thirds, quarters, fifths, etc. Have them graph the new results.
Have students try different brands of antacids to determine any results differences.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn how rocket thrust is generated with propellant. The two types of propellants are discussed—liquid and solid—and their relation to their use on rockets is investigated. Students learn why engineers need to know the different properties of propellants.

Students acquire a basic understanding of the science and engineering of space travel as well as a brief history of space exploration. They learn about the scientists and engineers who made space travel possible and briefly examine some famous space missions.

Through the continuing storyline of the Rockets unit, this lesson looks more closely at Spaceman Rohan, Spacewoman Tess, their daughter Maya, and their challenges with getting to space, setting up satellites, and exploring uncharted waters via a canoe. Students are introduced to the ideas of thrust,...

The purpose of this lesson is to teach students how a spacecraft gets from the surface of the Earth to Mars. Students first investigate rockets and how they are able to get us into space. Finally, the nature of an orbit is discussed as well as how orbits enable us to get from planet to planet — spec...
References
University of Idaho, Idaho Tech, Rocketry: An Effervescent Race. Accessed February 14, 2006. http://www.uidaho.edu/idahotech/lessons/rockets/antacid.html
Copyright
© 2006 by Regents of the University of ColoradoContributors
Jeff White; Brian Argrow; Luke Simmons; Jay Shah; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education, and National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: November 16, 2020






User Comments & Tips