Quick Look
Grade Level: 12 (10-12)
Time Required: 30 minutes
Expendable Cost/Group: US $2.50
Group Size: 2
Activity Dependency:
Subject Areas: Biology, Chemistry, Life Science, Physical Science, Physics
NGSS Performance Expectations:

| HS-PS2-6 |

Summary
Students test and observe the "self-cleaning" lotus effect using a lotus leaf and cloth treated with a synthetic lotus-like superhydrophobic coating. They also observe the Wenzel and Cassie Baxter wetting states by creating and manipulating condensation droplets on the leaf surface. They consider the real-life engineering applications for these amazing water-repellent and self-cleaning properties.Engineering Connection
Inspired by the lotus leaf, a revolution in self-cleaning surfaces is underway. Many researchers and engineers are developing synthetic superhydrophobic materials and products that mimic the lotus leaf's extremely water-repellent and self-cleaning properties. These materials and textiles would enable skyscraper windows and walls to never need human cleaning, not to mention an unlimited number of other objects: tents and awnings, painted houses, vehicle undercarriages, etc. Already in everyday use is clothing fabric that repels ketchup, mustard, red wine and coffee. Extreme water-shedding materials have the potential for applications in energy and medicine. Some technologists are developing self-deodorizing and disinfectant surfaces for bathrooms and hospitals, as well as similar technologies for keeping surfaces from fogging. Some technologies use the "lotus effect," whereas others employ the opposite property — superhydrophilicity. Future products may combine the two properties or use substances that can be switched back and forth to control the flow of liquids through microfluidic components.
Learning Objectives
After this activity, students should be able to:
- Describe the behavior of water on superhydrophobic surfaces.
- Define "lotus effect" and describe its water-repellent and self-cleaning properties.
- Explore how damage to Nano-TexTM cloth affects its "lotus effect" properties.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate scientific information (e.g., about phenomena and/or the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. Alignment agreement: | Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Chemical technologies provide a means for humans to alter or modify materials and to produce chemical products.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Develop a solution to a technological problem that has the least negative environmental and social impact.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
North Carolina - Science
-
Understand types, properties, and structure of matter.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 2 lotus leaf pieces, or other plant with a superhydrophobic surface (see note below); keep fresh leaves in a sealed plastic bag and refrigerated until ready to use
- 4 pieces of Nano-Tex™ cloth, 1 small (3 x 3 cm) and 3 larger (10 x 10 cm); see note below
- cup of water
- pipette
- dirt
- lab gloves
- tweezers
- ice cube
- Petri dish
- The Lotus Effect Worksheet, one per person
Note about leaves: The leaves of the following plants also demonstrate superhydrophobicity: broccoli, Brussels sprout, cabbage, collard greens, kale, taro (elephant's ear), tulip, turnip greens, and water lily. It is best if leaves are fresh, or have been kept refrigerated since being cut. Alternatively, purchase dried lotus leaves from an Asian grocery, although the dried leaves do not work well for the third section of the lab (Motion and the Lotus Leaf). Keep in mind that the oils from your hands will eventually destroy the effect; wear gloves if you plan to use the same leaf often.
Note about Nano-Tex™ cloth: Purchase garments made of Nano-Tex™ cloth at the stores listed at https://www.nanotex.com/. We used a $15 Ashworth Nano-Tex shirt purchased from Amazon. Other companies make products that demonstrate similar water- and stain-repellent effects.
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/duk_surfacetensionunit_act4] to print or download.Introduction/Motivation
(Begin with the associated lesson, and its Introduction/Motivation talk, to set the stage for conducting this activity with students. The lesson provides photos, short videos and background information on superhydrophobic surfaces, including condensation and the Wenzel and Cassie Baxter wetting states, and real-life engineering applications.)
How many of you have ever spilled something on yourself at lunch? If you do that at school, you just have to make do with a stain on your shirt until you get home. (Students probably have a few good stories to share about this.) What if you could make your clothes out of something that could not stain?...So whatever you spilled on yourself would just run right off and leave you clean and dry? Well today we are going to do just that.
Lotus plants (as well as some other plants) have a special surface on their leaves that allows water to flow right off of it without sticking, and take any dirt on the leaf off with it. This is an example of biomimicry — which is when we study a process or object in nature and figure out how to "mimic" its effect or properties with a synthetic product. Right now, scientists and engineers are learning how to copy the superhydrophobicity of lotus leaves and apply it to everything from clothing to outdoor paints.
(At this point, conduct the pre-lab questions activity as described in the Assessment section.)
In this lab, you will observe the superhydrophobicity phenomenon on a natural item and a synthetic material designed to mimic nature.
Procedure
Background
These are suggested procedures, which you may need to alter, depending on student level, time constraints, and availability of materials.
Before the Activity
- Cut pieces of lotus leaf (or other plant with a superhydrophobic surface). Seal fresh leaves in a plastic bag and refrigerate until ready to use.
- Cut pieces of Nano-Tex™ cloth.
- Gather materials and organize the lab stations.
- Make copies of The Lotus Effect Worksheet.
With the Students
Have students perform this lab at their desks, or group students into pairs to work at lab benches.
To guide students' lab investigations, hand out the worksheets, which include instructions and procedures, a table to record data and observations, and questions to answer as they go.
A. Pre-Lab
Place an ice cube in a small dish and lay a piece of a lotus leaf on top of it (see Figure 1). Set aside for Part C. While students are working on Part B, condensation will form on the leaf (see Figure 6).
B. Water and the Lotus Leaf
Have students perform the following experiments on the lotus leaf, recording their observations on the worksheets. Then, do the same using the piece of Nano-Tex™ cloth.
- Place a drop of water on it and observe its behavior (see Figure 2).
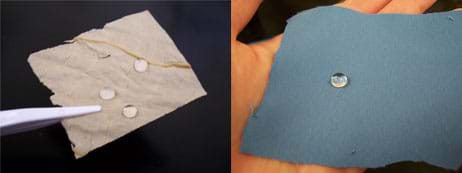
Figure 2. Dried lotus leaves (left) and Nano-Tex™ fabric (right) are both superhydrophobic. A water droplet on these surfaces "floats" on a cushion of air and easily rolls off the material. - Submerge it into a glass of water and observe its appearance (see Figure 3).

Figure 3. When a superhydrophobic surface is submerged, a silver sheen can be seen. This is caused by a thin layer of air trapped on its surface. - Observe the item after it is removed from the water (see Figure 4).

Figure 4. After being submerged, a superhydrophobic sheds any water immediately and emerges completely dry. - Sprinkle dirt (or pepper or glitter) on the item. Then place a few drops of water on it and tilt it so that water runs through the dirt (see Figure 5).
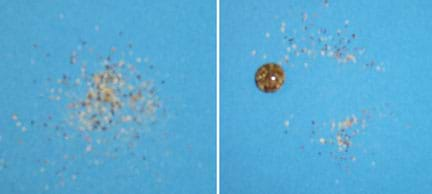
Figure 5. Demonstration of the self-cleaning lotus effect. As a water drop moves along the surface of a piece of Nano-Tex™ fabric sprinkled with pepper, it picks up the pepper flakes, but does not stick to the fabric. This effect helps the lotus (and a number of other plants) remain clean and free of parasites.
C. Motion and the Lotus Leaf
- Have students retrieve the lotus leaves on ice cubes that were set aside earlier.
- For this part of the experiment to work, at least a few water droplets about 1 mm in diameter must be on the leaf. If not, have students breathe gently on the lotus leaf and ice, as if trying to fog up a window. It may take a few breaths.
- Tilt the leaf to the side slowly. Expect the water droplets to stay in place (see Figure 6).
- Return the leaf to the horizontal orientation and introduce mechanical energy by using your other hand to gently tap the leaf or flick it with the tweezers. Watch the drops make the transition from the "sticky" Wenzel state to the superhydrophobic Cassie-Baxter state and bounce off the leaf.

Figure 6. When water vapor condenses on a lotus leaf (rather than falling on or being poured on), the water droplets behave differently. They form within the surface texture and become "pinned" to the leaf. They remain "stuck" to the leaf until mechanical energy is introduced to "unpin" the droplets.
D. Damage and the Lotus Effect
If you scratch the Nano-Tex™ cloth deep enough to make visible marks, the scratched area is no longer superhydrophobic and water sticks to the fabric at that spot (see Figure 7).
- Have student teams determine ways in which they can damage the larger pieces of cloth safely, and define "slightly," "moderately" and "highly" damaged.
- Have students label the three larger pieces of cloth "slightly," "moderately" and "highly" damaged and proceed to damage them accordingly.
- Have students pipette water onto each cloth and record how the damage affected the superhydrophobic ability of its ability to shed water.
- Have students describe situations in which the damaged cloth may be too fragile to be used.

Figure 7: When the surface of the cloth is scratched, its superhydrophobic properties can be damaged and water can bead on the surface.
Conclustion
- After the lab is finished, lead a class discussion comparing results and answers.
- Assign students to write answers on separate sheets of paper, to the two concluding questions of on the worksheet.
Assessment
Pre-Activity Assessment
Pre-Lab Questions: After the introductory talk, but before conducting the lab, have students write on note cards their answers to the following questions.
- How are hydrophobic and hydrophilic different?
- What is the lotus effect?
- How is the lotus flower's importance in Asian religions related to this effect?
Activity Embedded Assessment
Worksheet Observations: Circulate throughout the classroom, checking each student's observations, as recorded on the The Lotus Effect Worksheet table. Ask questions about student answers to gauge their comprehension, and help them gain clearer pictures of the concepts in the lab and their extensions to real-world engineering problems and products (such as self-cleaning, stain-repellent garments).
Post-Activity Assessment
Concluding Questions: Assign students to write answers (on separate sheets of paper), to the concluding questions of the lab.
- The Nano-Tex™ cloth is an example of biomimicry. The self-cleaning properties of the lotus plant were studied and a synthetic version of its surface was developed. When this treatment is added to fabric, the garments repel dirt and stains just like the lotus plant. What other products could benefit from a self-cleaning surface? Include at least three examples and explain how the self-cleaning ability would be especially useful for each application.
- Water droplets formed from dew or other condensation may act differently on supherhydrophobic surfaces than water poured or dropped on these surfaces. Does this limit the applications for self-cleaning surfaces? How could some products be modified to help self-cleaning products work even for water condensation? Write at least one paragraph to answer each question.
Share: Ask students to share what they learned about the lotus effect with their families.
Troubleshooting Tips
- Direct students to avoid handling the lotus leaf with their hands, as the oil from their skin destroys the superhydrophobic effect. Use lab gloves and/or tweezers.
- If using fresh cuttings, store them in a sealed plastic bag in a refrigerator until ready to use. The superhydrophobic effect lasts about three hours after the leaves are removed from the refrigerator.
- Instead of dirt, consider using pepper or glitter for the self-cleaning demonstration.
- For Part B, dried lotus leaves can be successfully used when students test the superhydrophobic properties of the leaf and Nano-Tex™ cloth. However, dried lotus leaves work poorly for the condensation demonstration in Part C.
- For Part C to work, the water droplets from condensation must be at least 1 mm or larger. If you have time and space, place the leaves on the ice cubes 1-2 hrs before class to give water droplets more time to form. As mentioned elsewhere, the size of the water droplets can also be increased by students breathing on the lotus leaves and ice cubes, as if trying to fog up a window or mirror.
Activity Extensions
Use plants and fabrics that are not superhydrophobic to show how water behaves differently on them.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to superhydrophobic surfaces and the "lotus effect." Students learn how plants create and use superhydrophobic surfaces in nature and how engineers have created human-made products that mimic the properties of these natural surfaces.

Through hands-on activities, students learn how the combination of adhesive forces and cohesive forces cause capillary motion. They study different effects of capillary motion and use capillary motion to measure surface tension. Students explore the phenomena of wetting and hydrophobic and hydrophil...

Students are presented with the concepts of wetting and contact angle. They are also introduced to the distinction between hydrophobic and hydrophilic surfaces. Students observe how different surfaces are used to maintain visibility under different conditions.
References
de Gennes, Pierre-Gilles, et al. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves. New York, NY: Springer, 2004.
Forbes, Peter. "Self-Cleaning Materials: Lotus Leaf-Inspired Nanotechnology." Published July 30, 2008. Scientific American. Accessed July 2010. http://www.scientificamerican.com/article/self-cleaning-materials/
Zenner, Greta M., et al. "Lotus Leaf Effect." University of Wisconsin-Madison Materials Research, Science and Engineering Center (MRSEC) Interdisciplinary Education Group, Nanoscale Informal Science Education Network. Accessed July 2010. http://mrsec.wisc.edu/Edetc/EExpo/surfaces/NanoSurfaces_ProgramGuide.pdf
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 Duke UniversityContributors
Jean Stave, Durham Public Schools, NC; Chuan-Hua Chen, Mechanical Engineering and Material Science, Pratt School of EngineeringSupporting Program
NSF CAREER Award and RET Program, Mechanical Engineering and Material Science, Pratt School of Engineering, Duke UniversityAcknowledgements
This digital library content was developed under an NSF CAREER Award (CBET- 08-46705) and an RET supplement (CBET-10-09869). However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: March 14, 2021





User Comments & Tips