Quick Look
Grade Level: 12 (10-12)
Time Required: 1 hours 15 minutes
Expendable Cost/Group: US $8.50 This activity requires some non-expendable items typically available in high school chemistry lab classrooms; see the Materials Lists for details.
Group Size: 3
Activity Dependency: None
Subject Areas: Biology, Chemistry, Physical Science, Physics
NGSS Performance Expectations:

| HS-PS1-3 |

Summary
Students observe capillary action in glass tubes of varying sizes. Then they use the capillary action to calculate the surface tension in each tube. They find the average surface tensions and calculate the statistical errors.Engineering Connection
The chemical engineering of many industrial processes depends on the accurate measurement of surface tension. When an object must be painted or coated with a material, the surface tension of the coating must be carefully maintained to produce the desired coating thickness without any unevenness. The strength and effectiveness of detergents are also partially determined by surface tension. One accurate way to measure surface tension is through capillary action.
Learning Objectives
After this activity, students should be able to:
- Describe how the combination of adhesive and cohesive forces causes water to rise in a thin tube (capillary action).
- Discuss the role of capillary action in measuring surface tension.
- Practice using scientific notation and calculating experimental error.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. Alignment agreement: Use mathematical representations of phenomena or design solutions to describe and/or support claims and/or explanations.Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: | The functions and properties of natural and designed objects and systems can be inferred from their overall structure, the way their components are shaped and used, and the molecular substructures of its various materials. Alignment agreement: |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use data from a sample survey to estimate a population mean or proportion; develop a margin of error through the use of simulation models for random sampling.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
North Carolina - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use data from a sample survey to estimate a population mean or proportion; develop a margin of error through the use of simulation models for random sampling.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
North Carolina - Science
-
Compare physical and chemical properties of various types of matter.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Explain the effects of forces (including weight, normal, tension and friction) on objects.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
For the introductory teacher demonstration:
- a can of paint, motor oil or other fluid
- a container into which you can pour the above fluid
Each group needs:
- 1 ring stand with support ring attached
- capillary tubes of various diameters; suggested sources: McMaster-Carr http://www.mcmaster.com/ or Vitrocom http://www.vitrocom.com/
- large Petri dish
- twist-ties
- water and dark food coloring
- ruler
- eye protection (glasses or goggles) for each student
- Measuring Surface Tension Worksheet, one per person
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/duk_surfacetensionunit_act2b] to print or download.Pre-Req Knowledge
Ability to manipulate algebraic equations, and calculate averages and standard deviations.
Introduction/Motivation
(Open a can of paint or motor oil or other fluid, and pour into a container or otherwise demonstrate its properties.)
What are some important properties of paint (or whatever liquid you are using)? What do we want to accomplish with the paint? What does it need to be able to do? (Encourage students to think about what would happen if paint spread like plain water on a wall. The surface tension is too high and you cannot get the paint to spread evenly. On the other hand, if the surface tension is too low, the paint layer will be too thin and the surface under the paint will show through. If any students have painting experience—artistic or home improvement or hobbies—encourage them to describe any problems they had that are related to this surface tension discussion.)
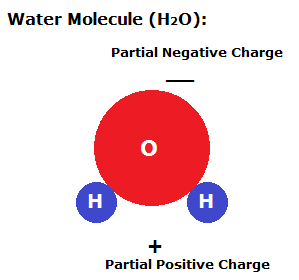
An adhesive force occurs when molecules of different types are attracted together. This occurs between water and glass, as the charged particles of water are attracted to the glass more than they are to each other. If particles prefer to be near glass than other water molecules, then what do you think will happen when water is near glass? (Answer: it will be attracted to the glass!)
For liquids used in coatings, their surface tensions are extremely important to get just right so they work correctly. One of the most accurate ways to measure surface tension is to use capillary action, the ability for a fluid to climb a thin tube. In this lab activity, we will use capillary tubes in order to measure the surface tension of water. Capillary action is caused the adhesive forces between the glass and water are greater than the water's cohesive forces. Because the water is attracted to the glass, it will begin to rise up the wall of the glass tube.
Procedure
Background
These procedures are written with advanced students in mind. They can be modified for use in a general class.
Before the Activity
- Gather materials and make copies of the Measuring Surface Tension Worksheet.
- Organize lab stations.
- Mix food coloring with water and distribute to each station.
- If lab time is limited, attach the capillary tubes to the ring stands above the Petri dishes before class (see Figure 1).

Figure 1: The height water will rise in a thin tube is determined by the material of the tube, the diameter of the tube, and the surface tension of the climbing liquid.
With the Students
- Divide the class into groups and send them to the lab stations.
- Direct students to follow the worksheet lab instructions, recording data and answering questions.
- Have students carefully attach the capillary tubes to the support rings on ring stands in increasing order of their inner diameters.
- Have groups each place a large petri dish beneath the capillary tubes and lower the tubes so that their ends are just above the bottom of the dish (see Figure 1).
- Have students fill the Petri dishes with dyed water and watch the water rise in each tube. Expect the water to reach its maximum height quickly (2-3 minutes).
- Have groups measure the height of the water in each tube and record their data.
- Have each student calculate the surface tension found experimentally for each tube.
- Have each student calculate the average surface tension overall and the standard deviation.
- The accepted value of the surface tension of water in air at 20 °C is γ = 0.073 J/m2. Have students compare their measured values for surface tension with the accepted values and speculate on why the values are different.
- Assign students to individually research a substances for their surface tensions and applications, as described in the Assessment section. Follow-up in a later class period to share findings.
Assessment
Pre-Activity Assessment
Brainstorming: In small groups have students agree upon three applications in which knowing the surface tension of a product is important in quality control. (Anywhere a liquid needs to adhere to a surface in a particular way will depend on surface tension. Examples include: Painting cars or aircraft, cleaning with soap and water, and applying sunscreen or moisturizer.)
Activity Embedded Assessment
Activity Questions: Students demonstrate their thought processes during the activity by answering the questions on the Measuring Surface Tension Worksheet. They extend their learning to the real-world problem of measuring the surface tension of a liquid to ensure proper application in an industrial process. Gauge student understanding by circulating the classroom and asking how they answered specific worksheet questions.
Exploring Further:
Post Assessment: Ask students the following questions to assess their understanding:
- What type of forces cause capillary action? (Answer: Electrical forces between molecules of water, known as adhesive forces.)
- What type of forces resiste capillary action? (Answer: Cohesive forces and gravity.)
- What would happen if the adhesive forces between water and the glass were even stronger? (Answer: Water in a glass tube would rise further up the sides of the glass tube.)
Exploring Further: Assign students to individually research the surface tensions reported online for various substances, and report on applications for which these substances are used.
Safety Issues
- Have students wear eye protection since glass is used in this experiment.
- Handle the capillary tubes with care! The tubes used in this experiment have a thick outer wall, but the glass is still fragile and can be easily broken if not treated gently.
Troubleshooting Tips
- Important: The measurement of surface tension depends greatly on: 1) the purity of the water, and 2) cleanliness of the capillary tubes. (See pg. 6 in Capillarity and Wetting Phenomena by Pierre-Gilles de Gennes, et al., for a short discussion.) Students' results should still agree within an order of magnitude with the accepted value, but will probably be less than the accepted value.
- To see the dyed water in the thinnest tubes, dye the water very dark. If students still have trouble seeing the water, suggest they place a piece of white paper directly behind the capillary tubes.
- Capillary tubes are fragile. Depending on students and time constraints, you may want to set up the capillary tubes and large Petri dish (without water) before class.
- Clean capillary tubes as soon as possible after class by submersing them in water and then blowing air through them.
- On occasion, water rises significantly different amounts, depending on which side of the capillary tube is placed in the water. If possible, check tubes before class or number the capillary tubes given to each group and note if any seem to behave oddly.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are presented with a short lesson on the difference between cohesive forces (the forces that hold water molecules together and create surface tension) and adhesive forces (the forces that causes water to "stick" to solid surfaces. Students are also introduced to examples of capillary action...

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Students are presented with the concepts of wetting and contact angle. They are also introduced to the distinction between hydrophobic and hydrophilic surfaces. Students observe how different surfaces are used to maintain visibility under different conditions.

Students are presented with the question: "Why does a liquid jet break up into droplets?" and introduced to its importance in inkjet printers. A discussion of cohesive forces and surface tension is included, as well as surface acting agents (surfactants) and their ability to weaken the surface tensi...
References
Adison, Arthur W., et al. Physical Chemistry of Surfaces. New York, NY: Wiley, 1997, p. 16-19.
Brown, Theodore, et al. Chemistry: The Central Science. 9th edition. Upper Saddle River, NJ: Pearson Education, Inc., 2003. (General information on surface tension and capillary action.)
de Gennes, Pierre-Gilles, et al. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves. New York, NY: Springer, 2004.
JRank Science & Philosophy Science Encyclopedia. "Capillary Action." Science.jrank.org. Accessed June 2010. http://science.jrank.org/pages/1182/Capillary-Action.html/
Mike. "Tree Physics 1: Capillary Action, the Height of Trees, and the Optimal Placement of Branches." Posted July 2009. Npand.wordpress.com. Accessed August 2009. (Derivation of water height in capillary tubes.) http://npand.wordpress.com/2008/08/05/tree-physics-1/.
Robinson, Clay. "Capillary Action." Last updated January 27, 2009. Accessed August 2009. (Includes discussion of capillary action in soil.) http://www.wtamu.edu/~crobinson/SoilWater/capillar.html
Smith, S. E. "What is Capillary Action?" Accessed June 2010. http://www.wisegeek.com/what-is-capillary-action.htm.
Stein, Becky. "Capillary Action." Last updated August 8, 2009. Chemwiki.ucdavis.edu. Accessed July 2010. http://chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of_Matter/Intermolecular_Forces/Cohesive_And_Adhesive_Forces/Capillary_Action.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 Duke UniversityContributors
Jean Stave, Durham Public Schools, NC; Chuan-Hua Chen, Mechanical Engineering and Material Science, Pratt School of EngineeringSupporting Program
NSF CAREER Award and RET Program, Mechanical Engineering and Material Science, Pratt School of Engineering, Duke UniversityAcknowledgements
This digital library content was developed under an NSF CAREER Award (CBET- 08-46705) and an RET supplement (CBET-10-09869). However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: March 5, 2021






User Comments & Tips