Quick Look
Grade Level: 10 (9-12)
Time Required: 45 minutes
Expendable Cost/Group: US $2.00 This activity also uses some non-expendable items such as lab equipment and marbles; see the Materials List.
Group Size: 3
Activity Dependency: None
Subject Areas: Chemistry, Earth and Space, Physical Science

Summary
Students learn about the underlying factors that can contribute to Plinian eruptions (which eject large amounts of pumice, gas and volcanic ash, and can result in significant death and destruction in the surrounding environment), versus more gentle, effusive eruptions. Students explore two concepts related to the explosiveness of volcanic eruptions, viscosity and the rate of degassing, by modelling the concepts with the use of simple materials. They experiment with three fluids of varying viscosities, and explore the concept of degassing as it relates to eruptions through experimentation with carbonated beverage cans. Finally, students reflect on how the scientific concepts covered in the activity connect to useful engineering applications, such as community evacuation planning and implementation, and mapping of safe living zones near volcanoes. A PowerPoint® presentation and student worksheet are provided.Engineering Connection
Volcanologists are geologists who study volcanic processes and eruptions. Though volcanologists focus on the fluid dynamics, geology, earth processes and other related concepts around volcanoes, their findings can provide valuable insights for engineering innovations, such as in the field of geochemical engineering or for technologies that involve fluids. Understanding volcanic eruptions can help people in nearby communities to stay safe in the event of an eruption and help avoid triggering eruptions, as some events involving gas drilling have been linked to setting off volcanic activity. Engineers must have a thorough understanding of volcanoes to pursue advances in hydrocarbon recovery (such as gas lift techniques in porous reservoirs) or the use of magma for geothermal applications (exploiting the high temperatures of magma bodies to provide heat to geothermal systems for heat and energy).
Learning Objectives
After this activity, students should be able to:
- Define and describe viscosity and explain how it relates to the amount of pressure exerted on bubbles rising through magma.
- Describe how the viscosity of magma relates to the rise speed of bubbles, as well as the ability of a bubble to expand in magma.
- Describe “low” and “high” viscosity fluids.
- Explain how magma viscosity relates to the explosiveness of volcanic eruptions.
- Explain how the rate of pressure change or degassing time can affect how effusive or explosive eruptions are.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Scientific inquiry is characterized by a common set of values that include: logical thinking, precision, open-mindedness, objectivity, skepticism, replicability of results, and honest and ethical reporting of findings.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
analyze physical and chemical properties of elements and compounds such as color, density, viscosity, buoyancy, boiling point, freezing point, conductivity, and reactivity;
(Grades
9 -
10)
More Details
Do you agree with this alignment?
-
describe how the macroscopic properties of a thermodynamic system such as temperature, specific heat, and pressure are related to the molecular level of matter, including kinetic or potential energy of atoms;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 2 filled and sealed carbonated beverage cans, such as cola or lemon-lime soda
- (optional) 1 timer, to measure 30 seconds or less; alternatively, students can use the second-hand on the classroom clock
- 3 identical 150-ml glass beakers; note that other-sized beakers, columns, graduated cylinders, small rectangular prisms, test tubes, etc., will also work—just make sure the containers are identical (for each group), each hold 100 ml of fluid and are large enough that students can drop a marble into each
- 3 marbles or small objects that can be dropped into the aforementioned containers; as an optional extension, make available additional small objects of differing size, mass or density to provide for additional experimentation
- 100 ml each of 3 fluids with varying viscosity; prepare the solutions in larger beakers (such as 250 ml or larger) and pour them into the 150 ml beakers; for example, consider using water and corn syrup combined in varying proportions by volume, such as:
- 100 ml corn syrup
- 50 ml corn syrup and 50 ml water
- 120 ml corn syrup boiled down to 100 ml to create more viscous corn syrup
- 3 plastic drinking straws, such as a 100-pack for $7 from Ikea/Amazon
- 3 glass stirring rods
- Viscosity and Pressure in Volcanic Eruptions Worksheet, one per student
To share with the entire class:
- (optional) projector to show the Volcano Presentation, a PowerPoint® file
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/rice_erruption_activity1] to print or download.Pre-Req Knowledge
Students should have a basic understanding of volcanoes and volcanic eruptions and be familiar with fluid behaviors in situations of flow and static equilibrium, understanding that these behaviors may vary based on temperature and fluid composition. Students should also be familiar with the concept of gas exerting pressure inside a closed container.
Introduction/Motivation
Today we are going to talk about something that we all know about, but that is still a great mystery to us in many ways. What do you know about volcanoes? (Listen to student answers. Expect students to answer with a general description of a volcano as a large landmass that is prone to eruptions.) Great! I heard you mention lava, magma, eruptions and heat. Today, we are going to delve into some characteristics that make each volcano unique by looking at certain features that cause them to behave differently.
Volcanoes have long fascinated humans with their immense—sometimes incredibly destructive—power and impact. Would any of you like to live near a volcano? (Expect students to respond by mentioning the dangers of being close to an active volcano, including the potential for death and destruction caused by eruptions.) So, we know the basics of volcanoes, but what makes them dangerous and why wouldn’t you want to live near one? (Heat, fire, hot magma, etc.) Commonly, people associate volcanoes with eruptions of a violent nature, but many volcanoes do not pose a catastrophic risk to their surrounding environments because they do not erupt violently.
Now let’s take a closer look at what makes a volcano dangerous. (Show or draw a basic diagram of a volcano, such as Figure 1, with a conduit that connects a magma chamber located below the Earth’s surface to a volcano’s crater above the surface). During some eruptions, lava, as well as volcanic ash, gas and rock fragments, are sent miles into the air and surrounding areas, posing a large environmental hazard.
Volcanoes are dangerous because of the extreme temperatures of the molten (melted) rock involved in eruptions. When the hot molten rock remains underneath the Earth’s surface, it is called magma; when the rock reaches the Earth’s surface, it is called lava. Magma is a combination of molten (melted) rock and dissolved gases (mainly water vapor) that flow from a magma crater close to the Earth’s surface. Complex processes happen inside magma, and that is our focus today.
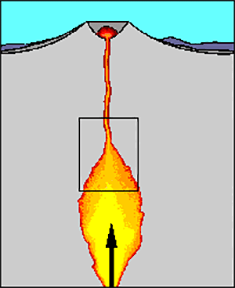
First, it is important to note that pressure is incredibly high under the surface of volcanoes. We measure pressure in units of Pascals, just like we measure length in meters. Because pressure is so high underneath volcanoes (in the range of 100 MegaPascals, more than 1,000 times more pressure than at sea level), volatiles are dissolved in magma while it remains at great depths below the Earth’s surface. However, as the magma rises towards the Earth’s surface, bubbles start to form and escape. At that point, a volcano can erupt in one of two main ways: an effusive eruption or an explosive eruption. Once magma reaches the Earth’s surface, lava can leave in river-like flowing streams, which we call effusive eruptions (show a photograph or draw on the classroom board: https://volcanoes.usgs.gov/vsc/glossary/effusive_eruption.html) or in violent bursts, which we call explosive eruptions (show a photograph or sketch on the board: https://volcanoes.usgs.gov/vsc/glossary/explosive_eruption.html; images also on slide 5).
Explosive eruptions pose huge dangers to nearby communities. Let’s consider the example of the 1991 eruption of Mount Pinatubo in the Philippines. After a series of initial steam explosions and earthquakes, Mount Pinatubo exploded, releasing fiery hot ash and that swept down 30+ miles of valleys, devastating towns and cities along the way—even reaching Clark Air Force Base, home to more than 15,000 American servicemen and dependents.
In order to understand how an explosive volcanic eruption works, it is important to know about the bubbles that come out of magma as pressure decreases when magma ascends to the Earth’s surface. An eruption’s explosiveness varies, depending on how thick the magma is. It is intuitive that thick fluids flow at a slower rate than thinner ones, but let’s explore the scientific term, viscosity, as a means of describing fluid thickness. Eruption intensity can also vary depending on how much pressure is inside the bubbles. We must understand these two concepts to understand violent volcanic eruptions and ultimately what we can do to engineer solutions to the inevitable hazards they pose.
Although the eruption of Mount Pinatubo resulted in many casualties and much damage, that outcome is considered a “success” on the part of the U.S. Geological Survey (USGS) and the Phillipine Institute of Volcanology and Seismology (PHIVOLCS). Upon recognition of Pinatobu’s activity, PHIVOLCS and USGS scientists initiated onsite monitoring for a few weeks. They conducted intensive studies of the volcano’s eruptive history and their analyses indicated that a large eruption was approaching. The team immediately issued urgent warnings that prompted the mass evacuation of people, aircraft and equipment to safe zones prior to Mount Pinatobu’s explosion. An estimated 5,000-20,000 lives were saved and at least $200 million in damages were averted due to the successful early warning and evacuation efforts. A thorough understanding of volcanoes made this feat possible in the form of engineered tools that monitored and predicted the volcanic eruption. Today we will learn about the fundamentals of what makes a violent eruption so catastrophic.
Procedure
Background
For many, the term “volcanic eruption” conjures images up of widespread disaster and destruction of entire towns and communities. In truth, eruptions come in a wide variety of forms and the explosive and damaging class is just one of a wide range of types. For example, many volcanoes in Hawaii are characterized by free-flowing magma, but rarely are as destructive as violent eruptions that spew large amounts of rock and ash. Mount St. Helens, one of the most well-known volcanoes in American history, is characterized by eruptions on the opposite end of the spectrum. The 1980 Mount St. Helens pyroclastic eruption killed 57 people and remains the most destructive in U.S. history.
In this activity, students explore two of the main concepts behind explosive eruptions (as opposed to the less-damaging, effusive eruptions): viscosity and the degassing rate. The first part of the activity is a simplified procedure based on a lab assay that is regularly performed by researchers who study fluid mechanics.
Before the Activity
- Gather materials and make copies of the Viscosity and Pressure in Volcanic Eruptions Worksheet.
- For each group, prepare and measure out 100 ml of each of the three fluids of varying viscosity into the 150-ml beakers labeled “A,” “B” and “C.”
- Make arrangements for use of an outside location to conduct Part 1, or else an indoor location that is easy to clean up after exploded soda cans.
- If available, set up a laptop and projector to show the 10-slide Volcano Presentation, a PowerPoint® file.
With the Students—Introduction
- Conduct the pre-activity assessment class discussion as described in the Assessment section.
- Present the Introduction/Motivation content, showing the presentation as you introduce the activity.
- Divide the class into groups of three students each.
- Hand out the worksheet.
With the Students—Part 1: Pressure Relief / Degassing
- To each group, hand out two filled and sealed carbonated beverage cans and a timer.
- Explain that when magma is “agitated” (that is, rises to the Earth’s surface), bubbles can form, which can lead to violent eruptions.
- Tell students that for this part of the activity, the cans represent volcanoes. Explain that they will agitate their “volcanoes.” Then they will open each can differently to model explosive versus effusive eruptions. Remind them to pay close attention to what happens so they can later describe and reflect on their observations. (Note: For the “eruption,” make sure that students are outside or open the cans on a surface that is easily cleaned.)
- Direct the students to agitate both of their soda cans for 10 seconds.
- For the “explosive” eruption can, completely open the can tab in less than one second.
- For the “effusive” eruption can, take 30 seconds to completely open the tab.
- Have students discuss within their teams the differences between each “eruption.” What was different about each situation? If students need help, encourage them to think about decompression rate—the time allowed for the cans to release gas and pressure.
- Direct students to fill out Part 1 of their worksheets.
With the Students—Part 2: Viscosity
- Explain the concept of viscosity and that higher viscosity magma usually lends itself to more explosive eruptions.
- Viscosity is the “thickness” of a fluid; the internal friction a substance has as it moves. The more viscous a fluid, the slower it is to flow.
- The more viscous magma is, the harder it is to move it. In other words, more force is required to shift thicker magma. When magma is flowing up through a conduit on its way to the Earth’s surface, intense pressures build up and the only relief from that pressure is at the exit of the volcanic crater. The more viscous a volcano’s magma, the more force is required to expel the magma (and the more pressure that builds up). Along with more viscous magma is a general trend towards more violent and “forceful” eruptions.
- Pass out the marbles and beakers A, B and C, one of each per group.
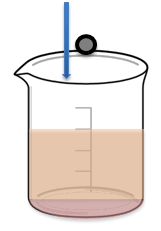
- Inform students that they have 10 minutes to rank on their worksheets the fluids in order of increasing viscosity. Explain that they need to back up their claims with data they gather based on the time it takes each marble to drop to the bottom of each beaker (see Figure 2).
- Direct students to use the straws and stirring rods to explore (and then explain) why more viscous magma might lend itself to more explosive eruptions. (Depending on your students, make this step as open-ended or guided as desired.)
- Straws: Think about the bubbles in magma. When magma rises towards the Earth’s surface, pressure decreases because less rock and earth are pushing down on the magma. As a result, volatiles once dissolved in the magma emerge as bubbles. (At this point, let students experiment with the straws and the liquids. Expect students to blow bubbles in beakers A, B and C, and observe the differing amount of force required to blow into each one and how that relates to viscosity. Hopefully, students lead themselves to this observation and realization prompted only by your explanation about the bubbles in magma in a volcano, but as necessary, provide guidance.)
- Stirring rods: Suggest that students use the applied physical force of stirring rods in beakers A, B and C to explore how the force of volcanic explosions is dependent on the magma viscosity. Expect students to stir the rods in each solution and observe how the force required to stir varies, depending on the viscosity. Encourage students to think about how this relates to effusive eruptions with flowing lava versus explosive eruptions with more viscous lava.
- Direct students to fill out Part 2 of their worksheets, including a detailed explanation of how viscosity and the amount of time a volcano is able to degas relate to how violent its eruption is.
- Have student share their explanations with the class; draw attention to exemplar responses. Ask them the reflection questions provided in the Assessment section.
Vocabulary/Definitions
degassing : The process of freeing gas from a solution. In the case of volcanoes, freeing gas from magma.
lava: Molten rock expelled from a volcano during an eruption. When rising magma from within a volcano reaches the Earth’s surface, it is called lava.
magma: A substance found below the Earth’s surface and inside volcanoes, composed of hot, liquid rock and dissolved gasses.
Plinian eruption: The largest and most violent type of volcanic eruptions. Usually associated with very viscous magma. Example: 1980 Mount. St. Helens eruption.
pressure: The continuous physical force exerted against an object by something that it is in physical contact with, in the case of erupting volcanoes, the gas in a bubble against magma.
pumice: A lightweight, rough and porous volcanic rock that is typically white or gray in color.
viscosity: A fluid’s resistance to flow or movement; describes internal friction of a fluid. A bubble encounters more resistance as it rises through honey than water.
volatiles: Chemical elements and compounds typically with low boiling points, such as nitrogen, water and carbon dioxide. They typically exist in the gaseous phase, but due to pressure underneath the Earth’s surface, in magma they are dissolved in small amounts.
volcanic crater: A typically bowl-shaped depression at the top of a volcano with a vent to below where magma reaches the Earth’s surface after traveling through a conduit.
volcanic eruption: The sudden occurrence of a discharge of steam and volcanic material through the vent/crater of a volcano.
Assessment
Pre-Activity Assessment
Discussion of Volcanic Eruptions: Engage students in an open discussion to help them to ponder why volcanoes can be dangerous. Encourage brainstorming and sharing of ideas. Ask students:
- What do you think causes volcanic eruptions? Why are they sometimes explosive?
- What are some everyday situations in which bubbles lead to or are involved in explosions? How might that relate to volcanic eruptions?
Activity Embedded Assessment
Worksheet: Have students fill out the corresponding sections of the Viscosity and Pressure in Volcanic Eruptions Worksheet as they work though Parts 1 and 2 of the activity. Review student answers to gauge their depth of understanding.
Lab Observations to Real-World Understanding: Ask students to predict how the carbonated beverage cans will react when agitated and opened. After they simulate explosive and effusive “eruptions,” have students describe how and why the eruptions differed and relate their observations to volcanoes and the gases that bubble out of rising magma. What exactly was it about how the cans were opened that meant the difference between an effusive and explosive eruption? (Answer: The critical difference was in the time allowed for the carbonation to degas from the can; that time difference [1 second versus 30 seconds] meant the difference between an explosive and effusive eruption. Fast degassing can cause violent volcanic eruptions; for example, an earthquake caused a large landslide on Mount St. Helens in May 1980, which suddenly exposed the volcano to lower pressures and led to an explosive eruption.)
Post-Activity Assessment
Reflection Questions: Individually or as a class, have students answer the following questions:
- What other concepts do you think affect the explosivity of volcanoes beyond what we have covered? (Possible answers: Magma composition; location of the volcano on the Earth’s surface in relation to fault lines, tectonic plates, etc.; volcanic activity; bubble characteristics in magma.)
- How do you think an understanding about the concepts covered in this activity connects to useful engineering applications? (Possible answers: Designing equipment to gather all sorts of data about volcanoes; providing global data on volcano activity; using available data to analyze and compare similar volcanoes; predicting future volcanic eruptions; city/town evacuation planning and implementation; mapping out safe living zones near volcanoes.)
Fluids Testing! For homework (or in class), have students carry out similar experiments using their own materials. For example:
- Have students use marbles, straws and glass stirring rods to test the viscosities of fluids and substances they find at home, such as juice, molasses, honey, cooking oil, melted butter and milk. Take it further by having students conduct their tests at varying temperatures.
- Have students try the same degassing procedure with carbonated beverages at different temperatures.
Safety Issues
Prepare for the explosive opening of agitated carbonated beverage cans by conducting Part 1 of the activity outside.
Activity Extensions
Change the temperature of fluids A, B and C, and see how their viscosities change.
Have students conduct the Measuring Viscosity activity so they can quantitatively calculate viscosity.
Have students complete the “Balloon Blow-Up” activity at http://om-annex.s3-website-us-west-2.amazonaws.com/science-explorer/balloon_blowup.html
Activity Scaling
- For lower grades, do just one of the two short activities or minimize the amount of testing students do with the fluids.
- For higher grades, have students conduct the Measuring Viscosity so they can calculate viscosity.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the causes, composition and types of volcanoes. They begin with an overview of the Earth's interior and how volcanoes form. Once students know how volcanoes function, they learn how engineers predict eruptions.

While learning about volcanoes, magma and lava flows, students learn about the properties of liquid movement, coming to understand viscosity and other factors that increase and decrease liquid flow. They also learn about lava composition and its risk to human settlements.

Students observe an in-classroom visual representation of a volcanic eruption. During the activity, students observe, measure and sketch the volcano, seeing how its behavior provides engineers with indicators used to predict an eruption.

Students are introduced to natural disasters and learn the difference between natural hazards and natural disasters.
References
“1980 Cataclysmic Eruption.” Last modified August 27, 2015. Mount St. Helens, Volcano Hazards Program, U.S. Geological Survey, U.S. Department of the Interior. Accessed February 2016. http://volcanoes.usgs.gov/volcanoes/st_helens/st_helens_geo_hist_99.html
Ball, Jessica. “Types of Volcanic Eruptions.” Geoscience News and Information, Geology.com. Accessed February 2016. http://geology.com/volcanoes/types-of-volcanic-eruptions/
“Why are some eruptions gentle and others violent?” Volcano World, Oregon State University. Accessed February 2016. http://volcano.oregonstate.edu/why-are-some-eruptions-gentle-and-others-violent
Copyright
© 2016 by Regents of the University of Colorado; original © 2015 Rice UniversityContributors
Nathan Truong; Austin Blaser; Thomas Giachetti; Helge GonnermannSupporting Program
Nanotechnology RET, Department of Earth Science, School Science and Technology, Rice UniversityAcknowledgements
This material was developed based upon work supported by the National Science Foundation under grant no. EEC 1406885—the Nanotechnology Research Experience for Teachers at the Rice University School Science and Technology in Houston, TX. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Last modified: August 16, 2023






User Comments & Tips