Quick Look
Grade Level: 11 (10-12)
Time Required: 2 hours
(two 60-minute sessions)
Expendable Cost/Group: US $10.00 Note: This activity also uses some non-expendable (reusable) supplies; see the Materials List for details.
Group Size: 3
Activity Dependency:
Subject Areas: Chemistry, Physics
NGSS Performance Expectations:

| HS-PS1-3 |
| HS-PS2-6 |

Summary
Students act as engineers to learn about the strengths of various epoxy-amine mixtures and observe the unique characteristics of different mixtures of epoxies and hardeners. Student groups make and optimize thermosets by combining two chemicals in exacting ratios to fabricate the strongest and/or most flexible thermoset possible.Engineering Connection
Engineers design more flexible thermosets by either an excess or lack of sufficient amine in their reactions. By learning how to make and optimize thermosets, students mirror chemical and polymer engineers who design new formulas for the multi-billion dollar plastics industry. When engineers make erasers, the mixture must be one that is very flexible. When engineers create car tires, they must be very strong. Every application requires a different working knowledge of how to formulate thermoset mixtures. Through this activity, students see the key role engineers play in the creation so many items that we rely upon every day.
Learning Objectives
After this activity, students should be able to:
- Determine what ratios of amine added to epoxy optimize flexibility and strength of the material and explain that strength has to do with the optimum stoichiometry.
- Describe how flexibility can be created in thermoplastics by ensuring that a less than optimum stoichiometric reaction takes place.
- Define a thermoset and thermoplastic.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: | Different patterns may be observed at each of the scales at which a system is studied and can provide evidence for causality in explanations of phenomena. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate scientific and technical information (e.g. about the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. Alignment agreement: | Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem. Alignment agreement: |
Common Core State Standards - Math
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Chemical technologies provide a means for humans to alter or modify materials and to produce chemical products.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Math
-
interpret information from various graphs, including line graphs, bar graphs, circle graphs, histograms, scatterplots, dot plots, stem-and-leaf plots, and box and whisker plots, to draw conclusions from the data and determine the strengths and weaknesses of conclusions;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
communicate mathematical ideas, reasoning, and their implications using multiple representations, including symbols, diagrams, graphs, and language as appropriate;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Texas - Science
-
describe how the macroscopic properties of a thermodynamic system such as temperature, specific heat, and pressure are related to the molecular level of matter, including kinetic or potential energy of atoms;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
analyze and explain everyday examples that illustrate the laws of thermodynamics, including the law of conservation of energy and the law of entropy.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 2 aluminum foil molds, ~ 12 mm X 8 mm X 100 mm, used to shape the epoxy for testing; make with standard aluminum foil (see the Procedure section)
- 2 paper cups
- felt tip pen
- 1 paper plate
- ~18 inches of heavy string
- 2 stirrers (see below)
- 2 - 3 ml syringes (see below)
- 2 - 12 ml syringes (see below)
- latex gloves, one pair per person
- safety glasses/goggles, one pair per person
- apron, one per person
- Thermoset Lab Worksheet, one per group
For class(s) to share:
- access to a fume hood
- 2 - 600 ml Pyrex® beakers for waste, such as this one for $11 each at https://www.flinnsci.com/beakers-borosilicate-glass-heavy-duty-600-ml/gp1049/
- slotted or hanging weight set, such as this one for $80 at https://www.flinnsci.com/slotted-weight-set-economy-choice/ob2067/ and https://www.flinnsci.com/hanger-slotted-weights/ap8856/
- 1-2 drying ovens, such as this one for $519 each at https://www.flinnsci.com/oven-laboratory-0.7-cubic-feet/ap1055/
- 100 wooden stirring sticks; such as these 100 for $1.50 at https://www.flinnsci.com/splints-wood-pkg.-of-100/ap4444/
- 2 tables or desks of equal height, for testing (see Figure 1)
- masking tape, few strips
- Thermoset Lab Presentation
- computer with internet connection and projection capabilities
Non-reusable supplies per class:
The following amounts provide enough material for one class of 30 students; multiple class periods require multiplying the quantities; in some cases, you may have materials left over.
- 250 g epoxy resin (Bisphenol A Diglycidyl Ether D.E.R. 332), for $44 at https://www.sigmaaldrich.com/catalog/product/sigma/31185?lang=en®ion=US
- 100 ml amine or hardener (Diethylenetriamine), for $33 at https://www.sigmaaldrich.com/catalog/product/sial/d93856?lang=en®ion=US
- 100 - 12 ml syringes without needles, for 95¢ each at https://www.flinnsci.com/syringe-without-needle-12-ml/ap1730/
- 100 - 3 ml syringes without needles, for 60¢ each at https://www.flinnsci.com/syringe-without-needle-3-ml/ap1728/
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/uoh_polymer_lesson01_activity1] to print or download.Pre-Req Knowledge
Students should have some chemistry background. This lesson could be taught as part of a high school chemistry course, or in another class while students are concurrently taking a high school chemistry course. It is also relevant for students who have previously completed a high school chemistry course.
Introduction/Motivation

What are plastics? (Listen to student ideas.) A plastic material is any of a wide range of synthetic or semi-synthetic solids that are moldable. Plastics are usually organic polymers of high molecular mass, and they often contain other substances.
Do you recall from our lesson the two types of plastics/polymers? (Listen to student answers.) The two types are thermoplastics and thermosetting polymers. And what is the difference between thermoplastics and thermosets? (Listen to student answers.) Thermoplastics are linear polymers whose final shapes can be changed through heating the material and melting it so that other shapes can be formed. So thermoplastics can be molded again and again, such as water and soda bottles. On the other hand, thermosets are polymers in which the final shapes of the products become set, or cured, due to irreversible chemical reactions. So thermosets can melt and take shape only once. Examples are tires and bumpers.
What sorts of plastics do you encounter every day? (Listen to student examples. Write them on the classroom board.) Thinking of all these examples, can you see how they are similar? They are all plastics! But they are all different from each other, too. What are some different physical properties of plastics? (Listen to student ideas.) Different properties include variations in and combinations of density, flexibility, strength and resistance to certain chemicals.
Have you ever wondered how plastics can be engineered to have such different properties? For example, pencil erasers and car tires are both thermosets, but they certainly have very different characteristics from one another. How do engineers do this?
Today, we will learn about the basics of polymers and then discuss two classes of polymers: thermoplastics and thermosets. In our activity, we mix amines and epoxies in varying ratios to simulate what polymer engineers do best: create plastics that are both strong and flexible for myriad applications.
Procedure
Notes for the Teacher
Activity recap: Each group pours thermosets into aluminum molds for two different epoxy-to-amine ratios and conducts flexure testing to determine how strong or flexible each thermoset can be when mixed. Students decide the optimum epoxy-to-amine ratios for increasing strength and the optimum epoxy-to-amine ratios for increasing flexibility. Results for all the groups' tests are tabulated, plotted and reviewed to determine the optimum amount of amine for both flexibility and strength.
Timing: Plan on 15 minutes for presentation and pre-lab questions, 35 minutes for the activity itself, and 5 minutes for clean-up.
Tip: If possible, do the presentation and pre-lab questions the day before in order to free up a little more time for the activity.
Background preparation: It is highly recommended that the teacher (as well as the students!) complete the Close Encounters of the Polymer Kind associated lesson before starting this activity.
Before the Activity
- Gather materials so everything needed is available before lab day.
- Make enough foil molds (or have students make them before or during the activity). To do this, form standard aluminum foil around a ~12 mm X 8 mm X 100 mm object and then gently extract the object without disturbing the foil mold shape. It takes 10 minutes for each group to make 2 molds, so consider having groups do this as a lab preparation project several days before the lab.
- Make copies of the Thermoset Lab Worksheet, one per group.
- Remind students the day before to come prepared with hair bands or clips. In addition, have a few elastic bands available for students to tie up loose hair before the activity.
- Pre-heat the oven(s) to 130 °F.
- Set up the Thermoset Lab Presentation to display in the classroom.
- Place latex gloves, goggles and aprons in an easy-to-find location.
- Place the 600-ml Pyrex® beaker for waste disposal in the fume hood in a water bath for cooling.
- Before each class, arrange group supplies in sets for easy access by students:
1 paper plate
2 paper cups
2 stirring sticks
2 - 3 ml syringes
2 aluminum foil molds
felt pen
1 - 10 ml syringe labeled epoxy at each table
1 - 3 ml syringe labeled amine at each table
2 filled paper cups, one labeled "epoxy" and the labeled "hardener"
- For Day 2 testing, set up two tables or desks as shown in Figure 1, making sure to maintain the correct overlap between the two tables to ensure consistency across all test samples. Make sure the tables are at the same height by adjusting the legs as needed. Place a strip of masking tape on each tabletop where you can later mark the exact placement of all the molds for consistency in the testing procedure across all groups.
With the Students
Lab Day 1: Making Thermosets (75-90 minutes)
Hand out a worksheet to each group.
Go through slides 1-6, guided by the script provided below and text in the "notes" section of each slide.
Slide 1 – Today, we will make thermosets and optimize the epoxy-to-amine (hardener) ratios just as engineers would do to ensure either flexible or strong systems, depending upon the application.
Slide 2 - How would you make a flexible chemical bridge? (Answer: Bridges with fewer chemical connections are more flexible.)
How would you make a strong chemical bridge? (Answer: Bridges with more chemical connections are stronger.)
Is there an optimum ratio? (Answer: Yes. Typically stoichiometry leads to superior mechanical properties.)
Slide 3 - Here we have two bridges. All things being equal, you could say that the bridge on the bottom is more mechanically robust than the bridge on the top. The obvious difference is the number of connections. The bridge with more connections is considered to be stronger.
Slide 4 – Thermoset materials undergo chemical reactions in which new covalent bonds are created. So let's look at some of the details. To make a linear molecule (thermoplastic), you could react molecule A with molecule B. One red group reacts with one blue group to make a purple group. No matter how much A and B react, they always create linear molecules (imagine many A and B molecules are mixed together). Next, let's consider the foundation for a thermoset system. Here we have small molecule A and small molecule D. These react and form E. As more and more react, they form this large branched, interconnected 3-D network. As discussed in associated lesson, if we want to get the strongest system, we need an exact stoichiometric system to get the maximum number of connections.
Slide 5 – Let's consider a real-life example. We have an epoxy-based molecule and amine based molecule. The amine can attack the epoxy forming crosslinks. Each hydrogen on the nitrogen is referred to as an "active" hydrogen—meaning it can participate in the reaction. The amine here has six active hydrogen molecules so it has a functionality of six, while the epoxy has two epoxide groups. Following the same ideas as before, we can vary the properties by varying the ratio of amine and epoxy, which is exactly what we are going to explore in our lab activity.
Slide 6 - We will pour thermosets into two molds, and after they have hardened, we will conduct flexure tests. Each thermoset will have a different epoxy-to-amine ratio and you will optimize the system much like engineers do in order to design epoxies that have good strength and flexibility.
Assign each group a letter A, B, C or D and direct them to pour two molds in the exact ratios specified on the data table per the procedures. Give students time to complete the pre-lab questions after the presentation (15 minutes total for presentation and questions).
Review with students the safety equipment and safety practices described in the Safety Issues section.
Day 1 Chemical Preparation
- Have each group gather (or make) two aluminum foil molds that will be used to shape the epoxy for testing.
- Add the epoxy and the hardener to the paper cup using the ASSIGNED syringes (identify one specifically for epoxy and another specifically for amine) in volume ratios that are assigned to your group. MAKE SURE YOU ARE USING THE EPOXY SYRINGE FOR MEASURING EPOXY AND AMINE HARDENER SYRINGE FOR MEASURING HARDENER. Mixing epoxy, amine or mixture syringes causes the plungers to become solidified.
- Each group (A, B, C or D) has two ratios to pour into molds, and therefore, will make two mixtures. (For example: Group A - ratio/mold 1 (designated as A1) in the worksheet data table). Use 10 ml of epoxy measured using a 12 ml syringe; add 0.5 ml of amine hardener measured with a 3 ml syringe for the 20:1 case.)
- Using the stirring stick, carefully mix the epoxy and amine hardener together until the solution looks homogeneous (3-5 minutes). DO NOT POUR THE MIXTURE INTO THE MOLD. FOLLOW STEPS 5-9 BELOW.
- Put a paper plate underneath your aluminum mold to catch any spills.
- Use a CLEAN 3 ml syringe, and carefully fill each aluminum foil mold with exactly 3 ml of mixed solution from Step 4.
- Place your molds in the drying oven at 130 °F for the time indicated for your group.
- Pour the excess product into the 600-ml Pyrex® waste beaker in a water bath in the fume hood. Discard your cup, paper plate and the 3 ml syringe into the designated waste container.
- Check the drying mold every 15 minutes until fully hardened and remove from the oven. (Expect this to take an hour.) The teacher makes sure all molds are not tacky after they are removed from the oven.
Lab Day 2: Flexure Testing
Begin Day 2 by displaying Slide 7 of the presentation and reading the "'notes" to the class (also provided below).
- Flexure testing – Carefully remove as much of the foil as possible from the molds and perform the flexure tests. Be careful not to crack the mold. Answer the worksheet questions in complete sentences.
- Make sure everyone is wearing safety goggles. Set up two tables or desks as shown in Figure 1, making sure to maintain the correct overlap between the two tables to ensure consistency across all test samples. Tie a string directly in the center of the mold place it across the gap between the two tables. Add weights to the string until the test sample breaks. Document your observations and the weight achieved before failure.
- Complete flexure testing, and record your data on the classroom board. On the day of flexure testing, conduct your flexure test and calculate your flexure weight using F = mass X gravity or F = mass X 9.8 m/s2.
- Calculate average values, and plot data and analyze the results. Share your data on the classroom board, and record the average flexure weight in the correct column of the data table.
- Finish the analysis and post-lab questions on the worksheet (as described in the Assessment section).
Mold Testing Procedure – Must Wear Safety Goggles!
- Verify that every mold is hardened to touch. If little or no amine is added, the epoxy will never cure and the mold must be disposed of correctly and not tested.
- Place the molds across the gap between the two tables with 5 mm of each end of the molds overlapping on each table. Mark on masking tape placed on the tables the exact mold placement to ensure a 5 mm overlap consistency in the testing procedure across all groups.
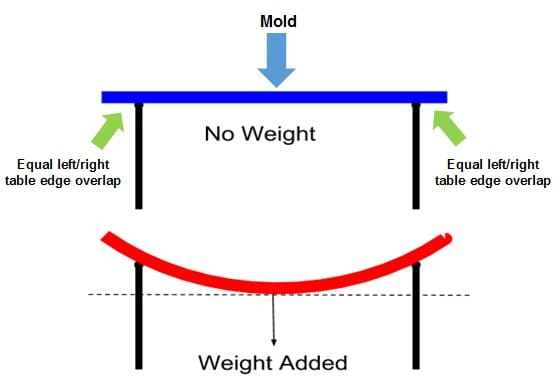
Figure 1. The testing setup. Place a mold across the gap between two tables with an equal amount of the mold resting on each tabletop. Then apply the weight. - Tie a string to the middle of the mold and attach a 100 g mass.
- Continue to SLOWLY add more weight: three more 100 g masses, then three 200 g masses, then four additional 500 g masses, followed by 1 kg mass until you reach the 10 kg mark or when the mold breaks or falls between the tables. Inform your teacher so s/he can verify that you have correctly followed the procedure. Expect the molds to break before you reach the 10 kg point, but not, record this as an observation and stop adding mass beyond the 10 kg point.
- Record the mass in the data table regardless of whether the mold bent between the tables and record whether the mold bent, broke or held. (Teacher note: If it held 10 kg and if the mold poured was too thick, then eliminate the data point.)
Vocabulary/Definitions
amine: Nitrogen-containing organic compounds that are derived from ammonia.
enthalpic interaction: Attraction and repulsion due to molecular forces, such as Van der Waals.
entropy: The amount of disorder in the system; can be used to discuss how many configurations a polymer chain can take.
epoxy: A molecule that contains an epoxide chemical group; typically used in thermoset.
hardener: A molecule that chemically reacts with the epoxide group on the epoxy to create a new covalent bond. Typically an amine or anhydride based molecule.
Newtonian fluid: A simple fluid in which the state of stress at any point is proportional to the time rate of strain at that point; the proportionality factor is the viscosity coefficient.
non-Newtonian fluid: A viscous fluid whose stress versus strain rate is non-linear. Example: polymer
physical entanglement: Entanglements at the molecular level that are not due to chemical bonds. A bowl of spaghetti, and how the individual pieces of pasta wrap around each other, is a good analogy of physical entanglement.
polymer: (poly)=many and (mer) = unit. A long chain of covalently bond atoms, primarily composed of carbon-carbon bonds in the backbone of the chain.
polymer chemistry: Organic chemistry concepts that are used to construct polymers.
polymer physics: Physics that govern the inter-/intra-actions of polymer chains.
shear thickening: The increasing of viscosity due to applied shear.
shear thinning: The decreasing of viscosity due to applied shear.
thermoplastic: A polymer system that consists of many linear polymer chains that are only held together by enthalpic interactions and physical entanglements (for example, a bowl of spaghetti).
thermoset: A polymer system that has gone through a curing reaction and is "set" in its final shape; a thermoset cannot be reshaped by heating.
viscosity: A measure of a materials resistance to flow; for example, honey is more viscous than water.
Assessment
Pre-Activity Assessment
Pre-Lab Questions: Ask students the following questions:
- Why is it possible to vary the mechanical properties of our material?
- Is the material you are making a thermoplastic or a thermoset?
- In your own words, what is a thermoplastic?
- In your own words, what is a thermoset?
- In a laboratory, it is common to use a mass ratio instead of a volume ratio. If the density of epoxy and amine is 1.1 and 0.9 grams/ml, respectively, how much volume of each do you need to make a 10 gram sample with a 10:1 epoxy to amine mass ratio?
- What ratios were assigned to your group?
- To get stiff materials what type of ratio(s) do you need (large or small or in between)?
- For flexible materials what type of ratio(s) do you need (small or large or in between)?
Activity Embedded Assessment
Data Table 1: Use the average data of other groups during all the periods testing during the day to fill out the final column of the data table provided on page 7 of the Thermoset Lab Worksheet.
Post-Activity Assessment
Analysis, Graph and Post-Lab Questions: Plot the flexure weight (N) versus the amount of amine in the system (ml) on the graph included on the Thermoset Lab Worksheet. Require students to answer the five assessment questions in complete sentences:
- Which ratio(s) of amine hardener is (are) the stiffest?
- Which ratio(s) of amine hardener is (are) the softest?
- What can you conclude about the amine hardener or what is the general trend between phr versus flexure weight (N)?
- Why does it follow that trend?
- Think back to the bridge analogy, why is there a difference in strength between ratios? What stoichiometric ratio between the epoxy and amine that corresponds with and why?
Engage students in a discussion about what ratios of amines-to-epoxy optimize strength and why that would correspond with an optimum stoichiometry; whereas, if one wants to manufacture more flexible thermosets one should use either an excess or lack of sufficient amine in ratio to the epoxy. Also, further discuss with students how the properties of the designed materials are a result of the attraction and repulsion forces at the atomic scale.
Defending a claim: Using scientific knowledge and student-generated evidence from the lab data, ask students to defend their claim with an explanation for why the pattern in the data is reasonable based on what is known about how the polymer forms in the given reaction. Students can use information about polymers provided in the Close Encounters of the Polymer Kind lesson and the “Chemical Bridges” slides provided in the Thermoset Lab Presentation to explain the pattern in the data and justification for the design of polymer materials with specific properties.
Safety Issues
This is a very messy (but engaging) activity. Students must wear latex gloves for safety, so make sure no students in the class are allergic to latex.
Before beginning, review the material safety data sheet (MSDS), as you would before any chemical lab. MSDS' are included with shipped chemical products.
The epoxy is a very viscous, sticky material that tends to get everywhere. The amine is corrosive and acts as an irritant when it touches skin. Thus, all students MUST wear safety goggles and gloves. Safety precautions and prevention of skin contact are commensurate to working with acids and bases, as in other experiments. If a student comes in contact with a chemical, have him/her immediately wash the affected area with soap and cool water.
The reaction between epoxy and amine is highly exothermic. For certain ratios of material, it is possible for the reaction to run away (a run-away reaction is one in which the heat from the reaction contributes to the reaction in a circular fashion until the material gets so hot it burns) and vaporize the remaining materials. Keep this in mind when determining how to dispose of waste from the activity. Individual bars are small enough to avoid this issue, but the large amount of mass that could be present in cumulative waste could result in the exothermic reaction, vaporizing some of the material and breaking the waste container. Therefore, DO NOT STORE EPOXY/AMINE WASTE IN PLASTIC CONTAINERs; instead, use a Pyrex beaker. To help counter the heat released, also place the container in a water bath.
Safety Equipment Needed
Safety goggles (immediately wash eyes thoroughly if chemical contact occurs)
Aprons (thoroughly wash out any substance on the aprons before reuse)
Latex or rubber gloves (if skin-chemical contact occurs, immediately wash the affected area with soap and water)
Safety Practices for Students
- Do NOT touch face or wipe eyes during the activity.
- Do NOT sit or lean on the table, as it could cause it to tip, spilling the chemical solutions.
- Before the activity begins, tie up long hair to prevent loose hair from coming in contact with the chemical product.
- When syringing, submerge only the tip of the syringe.
- Do NOT, at any time during the activity, remove goggles.
- Do NOT squirt the liquid or point the syringe towards anyone, including yourself.
- Dispose of waste in the Pyrex® beaker. Dispose of solid waste properly, as instructed by the teacher.
- At the end of the lab, wash your hands thoroughly with soap and water.
- The amine can very easily shoot out of the syringe, so be sure to very slowly draw up and slowly eject the amine. To help prevent splash, put your gloved hand around the syringe, and push slowly with your thumb on the plunger.
- The epoxy draws up slowly, perhaps with difficulty, in the syringe. As with the amine, very slowly draw up and slowly push out the epoxy.
- Leave your lab write-up away from the lab area, perhaps at your desk, so that chemicals do not get on the paper.
Troubleshooting Tips
- Make sure students are filling the molds with 3 ml and not spilling and cleaning up any spilled material. If the mold is overfilled with a full 10 ml instead of 3 ml, have students repeat the trial for that mold. If it leaks through a seam in the mold, students must re-do that mold trial.
- Make sure students are not mixing the epoxy and hardener plungers. If they do, replace the contaminated raw materials for the next groups at that station throughout the day.
- So as to not leave the oven on overnight, turn it off after the last class period of use and restart it in the morning.
- Leaving molds in the oven for a longer period of time will not present an issue.
Additional Multimedia Support
Three Visceoelastic Effects in the One Liquid video (1:06 minutes) shows the Barus Effect (extrudate swell), Weissenberg Effect (rod-climbing) and Kaye Effect (liquid leaping lanyards): https://www.youtube.com/watch?v=nX6GxoiCneY
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics.

Over several days, students learn about composites, including carbon-fiber-reinforced polymers, and their applications in modern life. This prepares students to be able to put data from an associated statistical analysis activity into context as they conduct meticulous statistical analyses to evalua...

Students explore the chemical identities of polymeric materials frequently used in their everyday lives. They learn how chemical composition affects the physical properties of the materials that they encounter and use frequently, as well as how cross-linking affects the properties of polymeric mater...
References
Colby, R. H., Boris, D. C., Krause, W. E., Dou, S. 2007. Shear thinning of unentangled flexible polymer liquids. Rheol Acta 46, pp. 569–575.
Hiemenz, Paul and Lodge, Timothy. Polymer Chemistry. Boca Raton, FL: CRC Press, 2007.
Copyright
© 2013 by Regents of the University of Colorado; original © 2012 University of HoustonContributors
Brian Rohde; Don McGowanSupporting Program
National Science Foundation GK-12 and Research Experience for Teachers (RET) Programs, University of HoustonAcknowledgements
This digital library content was developed by the University of Houston's College of Engineering under National Science Foundation GK-12 grant number DGE 0840889. However, these contents do not necessarily represent the policies of the NSF and you should not assume endorsement by the federal government.
Last modified: July 9, 2019





User Comments & Tips