Quick Look
Grade Level: 11 (9-12)
Time Required: 2 hours 15 minutes
(three class periods)
Lesson Dependency: None
Subject Areas: Chemistry, Physics
NGSS Performance Expectations:

| HS-PS2-6 |
Summary
Students are introduced to the multidisciplinary field of material science. Through a class demo and PowerPoint® presentation, they learn the basic classes of materials (metals, ceramics, polymers, composites) and how they differ from one another, considering concepts such as stress, strain, ductile, brittle, deformation and fracture. Practical examples help students understand how the materials are applied, and further information about specific research illustrates how materials and material science are useful in space exploration. A worksheet and quiz are provided.Engineering Connection
Creative engineering materials are continuously being developed, selected and used in all facets of industries, from consumer products to space exploration. Chemistry and physics are the backbone sciences in this field. Purposefully designed materials provide the means for modern products and tools to be built. Humanity has had a firm grasp of engineering materials and how to manipulate performance for centuries. However, until recently, we have not had the tools to fully understand the underlying mechanisms to such enhancements and provide optimized material solutions. Scientists and engineers develop and use basic principles to design new materials for different and ever-demanding applications. Materials design and behavior assessment is a function of mathematics, experimentation and a firm understanding of metallurgy and material science principles. Collaboratively, we are able to produce optimized materials that serve multiple functions within a given application and environment.
Learning Objectives
After this lesson, students should be able to:
- Describe basic structures of materials.
- Relate basic structures to four classes of materials.
- Identify modes of failure, mechanical behavior and relate to classes of materials.
- Explain materials development for engineering design requirements.
- Discuss various applications of materials and role in society.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate scientific and technical information (e.g. about the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. Alignment agreement: | Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
Matter and energy. The student knows that matter is composed of atoms and has chemical and physical properties. The student is expected to:
(Grade
8)
More Details
Do you agree with this alignment?
-
relate chemical properties of substances to the arrangement of their atoms or molecules;
(Grades
9 -
10)
More Details
Do you agree with this alignment?
-
Science concepts. The student knows that relationships exist between the structure and properties of matter. The student is expected to:
(Grades
9 -
10)
More Details
Do you agree with this alignment?
-
relate the physical and chemical behavior of an element, including bonding and classification, to its placement on the Periodic Table; and
(Grades
9 -
10)
More Details
Do you agree with this alignment?
-
Science concepts. The student knows concepts of force and motion evident in everyday life. The student is expected to:
(Grades
9 -
10)
More Details
Do you agree with this alignment?
-
explore technology applications of atomic, nuclear, and quantum phenomena such as nanotechnology, radiation therapy, diagnostic imaging, and nuclear power.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
develop and interpret free-body force diagrams; and
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Science concepts. The student knows how atoms form ionic, metallic, and covalent bonds. The student is expected to:
(Grades
10 -
12)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/uoh_matlsci_lesson01] to print or download.Pre-Req Knowledge
Basic chemistry or physics concepts, such as the effects of temperature on solubility, force concept and safe laboratory concepts.
Introduction/Motivation
(In advance, prepare to show students the 28-slide Introduction to Material Science and Engineering Presentation, a PowerPoint file, and gather materials to conduct a class demonstration. See materials and instructions, below. The presentation includes basic information regarding material classes, material applications, and material behavior and serves as a foundation for acquiring basic knowledge for the remainder of the lesson. Allow 20-30 minutes for the presentation. The class demo illustrates the different classes of materials and material behavior, and engages the students in a review of presentation content.)
What is material science? (Listen to student ideas.) If it weren't for material scientists and engineers, we would have never made it to the moon, nor would your laptop work!
What do I mean when I say "materials"? What are the different classes of materials? Why are they created? What are their characteristics? How do we test them? How are they used? That's what we're going to investigate today. Let's take a look.
(Show the attached PowerPoint presentation, or alternative teaching resources. Then conduct the class demo.)
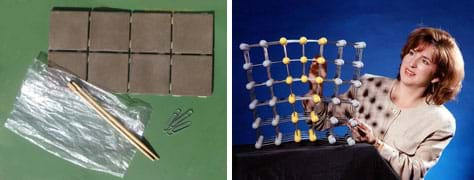
Materials List for Class Demo
- 1 ceramic tile or small plate (example ceramic)
- 1 Popsicle stick (example composite)
- 1 paper clip or copper electrical wire/tube (example metal)
- 2 plastic bags (example polymer)
- 1 hammer
- Demo Worksheet, one per student
(Hand out the worksheets to students and instruct them to fill in the answers during the demo.)
(Show students the tile, Popsicle stick, paper clip and plastic bag.) Which class of materials does each of these materials belong to? (Wait for a response, then proceed.)
(Bend the Popsicle stick slightly and release.) What type of deformation has occurred? (Continue bending until the wooden stick breaks.) Describe the failure of this material on your worksheet. Is it ductile, brittle or a combination? Did any permanent deformation occur?
(Bend the paper clip or copper wire until there is permanent deformation.) Why didn't the paper clip or wire break? (Continue bending the wire until it breaks.) Record your observations. What type of failure occurred?
(Show the ceramic tile to students and try bending it.) Will the ceramic permanently deform when I hit it with a hammer, or break?
(Place the ceramic tile into a plastic bag and seal the bag making sure all air is evacuated. Use the hammer to smash the ceramic tile until it breaks. Show students the remains and have them describe the failure on their worksheets.) What type of failure occurred?
(Show students the other plastic bag.) What type of deformation, if any, will occur if the bag is stretched? (Briefly stretch the bag until a small amount of permanent deformation is visible.) What type of deformation occurred?
(Continue stretching the bag until it tears.) What type of failure occurred?
(Have students complete the remaining questions on the worksheet. Then conduct the associated activity.)
Lesson Background and Concepts for Teachers
Material science has evolved during the last 40 years as different classes of materials became more and more competitive with one another. The four primary general classes of materials are metals, ceramics, polymers and composites. What makes these classes of materials unique is their composition, bonding and structure. Additionally, these distinct characteristics govern the applications for using each class of material. Following is a general summary of the four classes. Additional readings are suggested in the References section.
Material science is defined as the relationship of properties to its chemistry (composition) and structure. Understanding these relationships involves interdisciplinary knowledge of chemistry, physics and metallurgy. Provided engineers and scientists understand the type of atoms present and their arrangement, many properties may be understood and optimized for practical use. Chemical composition is fundamental in understanding any material type. Composition is the amount of an individual element that makes up a material. For example, steel is composed of iron and carbon and glass is composed of silicon, calcium, sodium, aluminum and oxygen. The structure can be defined as the arrangement of such elements (atoms), micro-features and macroscopic features.
Atomic structure is, as the name suggests, a particular arrangement of atoms and the electron structure. From chemistry, three primary forms of bonding exist: metallic, covalent and ionic. It is the atomic structure and interaction between elements that dictates which type of bonding prevails. Of course, additional bonding, such as hydrogen and London dispersion forces (van der Waals) exist and become important for polymers. For instance, metal alloys are strictly metallic bonding. Two metal elements with incomplete valence shells combine, filling the sub-valence shells, with remaining electrons present as a cloud. Interestingly, this electron arrangement is why metals are good electrical conductors. Ceramics are primarily bonded by strong covalent bonds and a few ionic bonds. The difference between the two is a sharing of valence electrons and transfer of valence electrons, respectively. The nature of these bonding types is why most ceramics are electrical insulators. Additionally, the nature of bonding is the source for many physical material properties and eventual mechanical properties. Bond strength is highly correlated to melting point, elastic stiffness and thermal expansion—meaning, the stronger the bonds the larger driving force is needed to separate such atoms.
For most solid materials, these arrangements are periodic and possess long-range order, with the exception of polymers and amorphous glasses. These periodic structures are called crystal structures and are present in seven primitive arrangements: cubic, hexagonal, triclinic, monoclinic, trigonal, tetragonal, and orthorhombic. These "lattices" are what make up the microstructure. Also, additional packing sequences that exist within each primitive arrangement create a total of 14 unit cells; most notable are body-centered cubic (BCC) and face-centered cubic (FCC). In addition to atomic bonding, these long-range atomic arrangements dictate inherent physical/mechanical properties and differentiate materials. For instance, BCC iron is much less ductile than FCC copper. The microstructure also includes larger features such as an array of crystals or grains, or a multitude of different solid phases. These features can be viewed using optical methods that do not require diffraction techniques or high magnification.
The macrostructure is the length scale that is comprised of a collection of microstructure features that show distinct characteristics. For example, collections of grains in a metal can be viewed at low magnification (x10) and appear to be flow lines or stripes. This length scale can provide very important details and can influence mechanical behavior of materials. A banded structure of steel can provide insights to fabrication methods and material strength. All of these length scales and distinct material features collectively make a material strong, fracture resistant, corrosion resistant, temperature resistant and ductile. However, no one material can have all of these wonderful attributes.
Pure Metals and Metal Alloys: Metal alloys are mixtures of two or more metallic elements. As mentioned earlier, metals are unique in that they are excellent electrical and thermal conductors. These physical properties are due to the nature of metallic bonding. Some metals also possess magnetic properties. Metals also possess a large capacity of mechanical deformation (aka ductility). Some metal bars can be shaped easily at room temperature. Most metals also have distinct strength levels: a yield stress (YS), and ultimate stress (UTS) and a fracture stress (FS), which collectively add to an entire mechanical response known as stress-strain behavior. To facilitate strength manipulation, metals have the capacity to be altered through mechanical, thermal or thermal mechanical treatments to achieve desired properties. Steel heat treatment for a hammer is different from that of a nail.
Ceramics: Ceramics are typically covalent-bonded solids that have very high melting points and stiffness. However, typically these materials are regarded as brittle. In contrast to metals and alloys, ceramics typically do not have distinct YSs and UTSs, but do have FSs. Ceramics are also useful in super-high temperature and corrosive environments. One advantage to the chemical compositions and bonding nature of ceramics is their inertness to many different corrosive media and oxidation environments. High temperature applications are suitable because of high strength retention at operating temperatures. Typical metals are too soft to sustain any mechanical loads at temperatures greater than 0.5 the melting point. Because of high stiffness, most ceramics are inherently hard and therefore abrasion- and wear-resistant. The hardest materials known are high-directional, covalent-bonded ceramics.
Polymers: Polymers are combinations of long-chained, covalent-bonded atoms that are mutually attracted by weaker bonding forces. Classic examples of polymers—car tires, Ziploc® bags, Kevlar®, glue and plastic water bottles—show the range of polymers that are manufactured and the variety of mechanical properties. Typically, polymers vary from very flexible, ductile materials to very hard and brittle materials. Polymers and their structures are dependent on the chemical composition of the base material and any fillers, extenders and plasticizers. Additionally, chemical composition also determines the degree of crystallinity in polymers. Typically, polymer chains are completely random and tangled. Depending on the chemistry, additives and stress state, these chains can obtain some periodic arrangement. Although, this arrangement is not counted as long-range order, polymers may possess short-range order.
Composite Materials: Composites are very common and not as scientific as one would think. Since ancient times, clay and straw have been mixed together to improve brick strength. Concrete, plywood, fiberglass and steel rebar are all common materials that are categorized as composites. In general, functional engineering materials for specific applications involve making composites from metal-ceramic, ceramic-ceramic, polymer-metal and polymer-polymer combinations. Depending on the application and properties required, different types of composite are selected. Composites allow for engineers and scientists to achieve unique property combinations that individual materials could not achieve. For instance, embedding ceramic particles in an aluminum or copper matrix improves both flexural strength and wear resistance, while maintaining a particular degree of toughening. However, aluminum alone is not very wear resistant and the ceramic has poor toughness. These unique properties are attractive and may not be provided by conventional materials.
All classes of material collectively account for all tangible objects on Earth. The field of material science enables the understanding and improvement of existing and new materials with the potential to catapult humanity to new frontiers.
The wealth of information in this lesson is adequate to intrigue students to consider pursuing paths of study in material science. The introductory presentation introduces the general classes of materials, terminology and applications. In general, students enjoy watching things break, especially if hammers are involved. How perfect, then, to introduce them to material science through the demonstration, as a way to reinforce the concepts relating all four material classes. Conducting the associated activity, Battle of the Beams, provides students with more insight into composite structures, while they apply concepts, and fabricate and test beams.
Associated Activities
- Battle of the Beams - Students explore the properties of composites to see how different materials and processing techniques affect material properties and performance. They create beams using Laffy Taffy and water, along with options for reinforcements (pasta, rice, candy drops) and fabricating temperatures. Teams compete for the highest strength beam, measured by flexure strength three-point bend tests and calculations.
Vocabulary/Definitions
amorphous: A solid state phase that lacks any long-range periodic order and may lack significant short-range order.
body-centered cubic: Closed packed cubic atomic arrangement in which atoms are located on each cube corner and one atom in cube center.
Bravais lattice: Three-dimensional geometric arrangement of atoms or molecules or ions composing a crystal.
brittle: Ability of a material to break, snap, crack or fail easily when subjected to external loads.
ductility: Ability of a material to undergo permanent deformation through cross-section reductions and elongation without fracture.
elastic deformation: Reversible alteration of the form or dimensions of a solid body under stress.
face-centered cubic: Closed packed cubic atomic arrangement in which atoms are located on each cube corner and one atom located at the cube face centers.
fracture strength: Strength of material at fracture.
mechanical behavior: Behavior of materials when subjected to external mechanical loads.
metallurgy: A branch of science that deals with the properties of metals.
plastic deformation: Irreversible alteration of the form or dimension of a solid body under stress.
strain: Describes displacement of particles in a deforming body. Commonly represented by ratio of length changed and initial length (engineering strain). delta L / L = e
stress: Description of force exerted on an object over a defined cross-sectional area. Stress = Force/Area
toughness: Able to withstand great strain without tearing or cracking.
ultimate tensile strength: Measured stress at the onset of necking. Graphically represents the highest stress on stress-strain curve.
yield strength: Measured stress at the onset of plastic deformation.
Young's modulus: Ratio of stress/strain. A measure of material stiffness.
Assessment
Worksheet: During the teacher-led class demo, have students fill in the answers on the Demo Worksheet, and answer the remaining questions after the demo is over. Review students' answers to gauge their comprehension of the subject. Have students keep the worksheets handy for reference during the associated activity.
Post-Lesson Quiz: Administer the attached Material Science Quiz, composed of 18 multiple-choice questions covering the basic aspects of material science topics covered in this lesson. Alternatively, administer the quiz after students complete the associated activity. Review students' answers to gauge their comprehension of the subject.
Research Paper: Assign students to each select a material or material system from any of the four classes of materials, research the material and write four-page double-spaced reports about its uniqueness and relevant applications. Require papers to include the following (with corresponding points given):
- Selection of a real material or material system (5 pts)
- Accurate identification of material class (5 pts)
- List of pertinent physical and mechanical properties (20 pts)
- List of three relevant applications in which the selected material is used (20 pts)
- A discussion relating physical and mechanical properties to selected applications, including justifications for why the material is used (30 pts)
- Complete sentences and proper grammar (5 pts)
- Well organized with a continuous flow of thought (10 pts)
- Four pages long, double-spaced (5 pts)
Additional Multimedia Support
Stronger-Smaller-Cleaner-Smarter: Making Stuff Activity Guide, Making Stuff: Education and Outreach. NOVA beta, PBS Online, WGBH Educational Foundation. Accessed March 22, 2012. (Author-recommended additional resources for material science reviews and introduction videos) http://www.pbs.org/wgbh/nova/education/making-stuff.html
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics.

Students create beams using Laffy Taffy and water, and a choice of various reinforcements (pasta, rice, candies) and fabricating temperatures. Student groups compete for the highest strength beam and measure flexure strength with three-point bend tests and calculations.

Over several days, students learn about composites, including carbon-fiber-reinforced polymers, and their applications in modern life. This prepares students to be able to put data from an associated statistical analysis activity into context as they conduct meticulous statistical analyses to evalua...
References
Carter, Giles F. and Donald E. Paul. Materials Science & Engineering. ASM International, December 2006.
Hertzberg, Richard W. Deformation and Fracture Mechanics of Engineering Materials. 4th edition. New York, NY: John Wiley & Sons, Inc., 1996.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 University of HoustonContributors
Marc BirdSupporting Program
National Science Foundation GK-12 and Research Experience for Teachers (RET) Programs, University of HoustonAcknowledgements
This digital library content was developed by the University of Houston's College of Engineering under National Science Foundation GK-12 grant number DGE-0840889. However, these contents do not necessarily represent the policies of the NSF and you should not assume endorsement by the federal government.
Last modified: February 25, 2020





User Comments & Tips