Quick Look
Grade Level: 5 (4-6)
Time Required: 2 hours
(two 60-minute class periods)
Expendable Cost/Group: US $20.00 This activity uses non-expendable (reusable) materials; see the Materials List for details.
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Measurement, Science and Technology
NGSS Performance Expectations:

| 5-PS1-1 |

Summary
Students become engineers as they create thermometers that can be used to monitor temperatures in reptile terrariums. By using liquid crystals, students learn about the characteristics of materials and wavelengths of light emissions. Students then apply this knowledge to make a liquid crystal thermometer and test it at various temperatures. Students then iterate their thermometer designs by using different mixtures of the liquid crystal formula to optimize the temperature range for their project’s environmental needs.Engineering Connection
Students directly observe color changes that indicate changes at the nanoscale level. These observations relate to work engineers are doing with silicon nanocrystals: using the wavelength of the light emissions as an indicator of the size of the crystal. The liquid crystal allows engineers to observe changes due to changes in size, which is due to the helix structure of the crystals being stretched and compacted. Making these observations allows engineers to iterate and design better products that use unique substances.
Learning Objectives
After this activity, students should be able to:
- Explain how liquid crystals can indicate temperature change.
- Calculate approximate temperature based on the color expressed by the liquid crystals.
- Define the difference between macro and nano scales.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-PS1-1. Develop a model to describe that matter is made of particles too small to be seen. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to describe phenomena. Alignment agreement: | Matter of any type can be subdivided into particles that are too small to see, but even then the matter still exists and can be detected by other means. A model showing that gases are made from matter particles that are too small to see and are moving freely around in space can explain many observations, including the inflation and shape of a balloon and the effects of air on larger particles or objects. Alignment agreement: | Natural objects exist from the very small to the immensely large. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Apply a product, system, or process developed for one setting to another setting.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
describe, plan, and implement simple experimental investigations testing one variable;
(Grade
5)
More Details
Do you agree with this alignment?
-
collect information by detailed observations and accurate measuring;
(Grade
5)
More Details
Do you agree with this alignment?
-
construct appropriate simple graphs, tables, maps, and charts using technology, including computers, to organize, examine, and evaluate information.
(Grade
5)
More Details
Do you agree with this alignment?
-
collect, record, and analyze information using tools, including calculators, microscopes, cameras, computers, hand lenses, metric rulers, Celsius thermometers, prisms, mirrors, pan balances, triple beam balances, spring scales, graduated cylinders, beakers, hot plates, meter sticks, magnets, collecting nets, and notebooks; timing devices, including clocks and stopwatches; and materials to support observations of habitats or organisms such as terrariums and aquariums; and
(Grade
5)
More Details
Do you agree with this alignment?
-
use safety equipment, including safety goggles and gloves.
(Grade
5)
More Details
Do you agree with this alignment?
-
identify changes that can occur in the physical properties of the ingredients of solutions such as dissolving salt in water or adding lemon juice to water.
(Grade
5)
More Details
Do you agree with this alignment?
-
explore the uses of energy, including mechanical, light, thermal, electrical, and sound energy;
(Grade
5)
More Details
Do you agree with this alignment?
-
demonstrate that light travels in a straight line until it strikes an object or travels through one medium to another and demonstrate that light can be reflected such as the use of mirrors or other shiny surfaces and refracted such as the appearance of an object when observed through water; and
(Grade
5)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- safety goggles
- safety gloves
- funnel
- digital scale
- weigh paper
- three small 20 ml glass vials
- cholesteryl oleyl carbonate 0.65 g per group, 25 g packs available online
- cholestreryl pelargonate 0.25 g per group, 100 g available online
- cholesteryl benzonate 0.10 g per group, 25 g available online
- clear contact paper (50 cm x 50 cm), two squares
- scissors
- small metal spatula, paintbrush, or popsicle sticks
- Liquid Crystal Lab Student Sheet, one per student
To share with the entire class:
- hot plate or heat gun
Additional “Before the Activity” materials:
For each student:
- one small glass mason jar
- kitchen grade string or twine
- pencil or popsicle stick
- Rock Candy Instructions and Observations Sheet
To share with the entire class:
- sugar
- water
- food coloring
- a pot (or sterile beaker)
- hot plate or stove
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/uot-2420-liquid-crystal-thermometers-design] to print or download.Pre-Req Knowledge
Students need a basic understanding of using a scale to measure the materials as well as an understanding of how to read a thermometer. They should have a general knowledge of how to set up a data table and record observations. Students should have a basic understanding of how color and light work.
Introduction/Motivation
Most reptiles require a terrarium environment that is between 24°C - 29° C on average during daytime hours. A stable temperature is crucial to their well-being and survival because they are cold-blooded and depend on their environment to control their body temperature. This is why you might see a lizard basking in the sun like a sunbather. However, lizards are also can be very curious and active creatures and the temperature needs to be monitored without adding in potential risks such as broken glass from a glass thermometer. So, how can you take the temperature reliably without glass and without intruding on their environment? Liquid crystals, which have been used in items like mood rings, have proven to be a safe and reliable thermometer for our cold-blooded friends.
Liquid crystals are sensitive to heat or temperature changes and are classified as an in-between phase between a crystalline solid and a liquid state. In other words, they show characteristics of both! Can you think of something else in science that shows characteristics of two different categories?
Procedure
Background
The way a material behaves on the macroscale is affected by its structure on the nanoscale. One way to observe nanoscale changes is with light waves and their corresponding color reflection. Materials behave differently on the nanoscale than larger materials due to surface area and the small size. Liquid crystal is considered a minor state of matter or in between phases. The most familiar use for liquid crystals are in mood rings, color-changing mugs, and thermometers for terrariums or screen doors.
Before the Activity
- Note: this pre-activity needs 10 days for the crystals to form.
- Gather materials; make copies of the Liquid Crystal Lab Student Sheet, enough for one per student.
- Depending on the students’ age group or familiarity with lab procedures, you may want to go through the entire procedure (as preparation for demonstrating the procedure to students).
Before the Activity - Rock Candy Activity
- Have students make rock candy to as an intro to a discussion on crystalline formation and self-assembly. Distribute copies of the Rock Candy Instructions and Observations Sheet.
- Gather the following materials; small glass jar (mason jars work well), kitchen grade string or twine, pencil or popsicle stick, sugar, water, and food coloring.
- In a pot (or sterile beaker), boil water and stir in sugar until the solution is saturated. Use about a 2:1 sugar-to-water ratio.
- After the sugar is completely dissolved, carefully pour the solution into the mason jar. Leave 2.5 cm of space from the solution to the rim of the jar.
- At this time, allow students to add food coloring to the sugar water and stir.
- Have students tie a string to a pencil or popsicle stick so that it will be about 13 mm from the bottom of the jar when balanced on the top of the jar. See the Figure 1 below for reference.
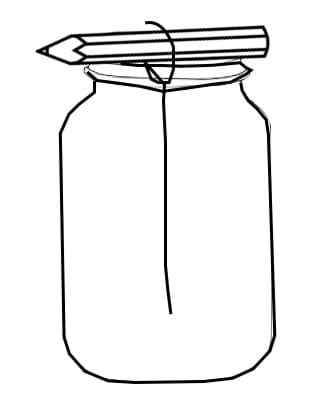
- Referring to Figure 2: Place the jar in a spot where it will be undisturbed for about 10 days. You should see sugar crystals starting to form within a couple of hours. If after 24 hours you still observe no crystals forming, dissolve additional sugar into your water. You can place a coffee filter, paper towel, or aluminum foil over the top of the jar to prevent dust and other contaminants in to the sugar water solution.
- If a hard layer of sugar crystals form on top of the jar simply break them up with a stick or similar instrument.
- Tell students to record observations and measurements daily using the Rock Candy Instructions and Observations Sheet.
- After pulling the string with the sugar crystals out of the jar, have students dry them on a paper towel.

With the Students
Day 1: Liquid Crystal Synthesis
- Distribute the Liquid Crystal Lab Student Sheet to students, and introduce the activity (see the Introduction/Motivation section). Students should take necessary lab safety precautions at this point and participate in safety briefing.
- Pass out the following materials for measuring; weight paper, scale, cholesteryl oleyl carbonate, cholestreryl pelargonate, cholesteryl benzonate, small glass vial, and small spatula to each group.
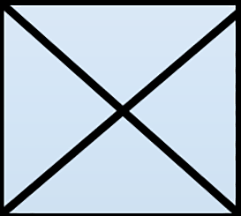
- Depending on age group or familiarity with lab procedures it might be good to demonstrate the entire procedure before having them work in groups. To start, demonstrate how to fold the weigh paper into a triangle one direction then unfold it. Next, fold it into a triangle the other direction creating an ‘X’ on the weigh paper that will act as a makeshift bowl for measuring the materials (Figure 3).
- Show students where to place the weigh paper on the scale and how to zero out the mass.
- Using the small spatula measure the following amounts and empty them the small glass vials: *Remember to either clean off the spatula or use a different spatula for each cholesteryl. Another option is to use a popsicle stick and throw them away after use.
- cholesteryl oleyl carbonate- 0.65g
- cholestreryl pelargonate- 0.25g
- cholesteryl benzonate- 0.10g
- Place the small glass containers in a heat resistant container filled with 1 cup of water. Place the container on a hot plate until the material in the vials melt completely; if you are using a hot plate with temperature controls, set it at 150°C and the materials will melt in about two minutes. See Figure 4 as an example setup on melting. If you do not have access to a hot plate, using a hair dryer on a hot setting for about 8 minutes will also melt the materials. A heat gun will only take about 30 seconds to melt the materials. Note the safety concerns and choose which is best for your students.
- Once the samples melt and are the consistency of honey, transfer them into smaller vials or directly on to the contact paper.

Day 1: Liquid Crystal Thermometer
- Cut 2 squares (5 cm x 5 cm) of contact paper.
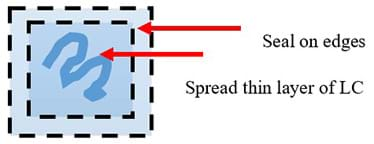
- Using the small spatula, transfer a thin layer of the liquid crystal into the center of the contact paper square.
- Place the second square on top and seal the edges of the two contact paper squares together to prevent the liquid crystal from seeping out the sides. See Figure 5.
Days 1-2: Experimentation
- Students can then test if the color change is easier to detect on a white or black background (If you want to skip this step you can transfer the liquid crystals directly onto black card stock and then laminate the paper. (Do NOT laminate in a hot laminator.) This is an easier option for younger students who might struggle with the contact paper. Note: self-stick lamination paper works great as well, but it more expensive.
- Another option for experimentation: students can use a water bath with a thermometer and determine the range of temperatures at which the liquid crystal can detect. What temperature is the highest that a color change can be observed and what temperature is the lowest that a color change can be observed?
- Students can record the various colors observed and the temperature range for each color in a data chart including the sequence of colors observed.
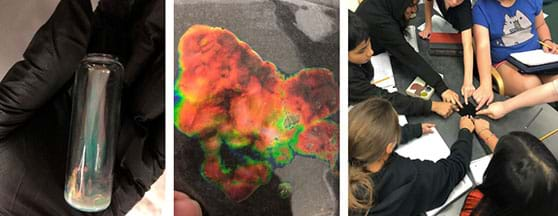
- Students can then modify the amount of one of the three compounds to see if they can adjust the range of observable temperatures of the liquid crystals. In order to conserve materials have them stay within a 10-20% of the original masses.
Day 2: Product Design
- Students can do research to determine what liquid crystals are currently used for various products, such as thermometers and commercial products like color changing mugs, etc.
- Students can design a product that incorporates liquid crystal and include a 2D drawing with measurement labels, a prototype, an advertisement for their product, and a small presentation to the class demonstrating their products use.
Vocabulary/Definitions
crystal: A piece of a solid substance having a natural geometrically regular form with symmetry.
liquid crystal : A substance that flows like a liquid but has some degree of ordering in the arrangement of its molecules.
macro: Large-scale.
nano: Very small- no larger than 100 nm in size.
self-assembly: A process in which a disordered system of pre-existing components forms an organized structure or pattern.
thermal: Heat energy.
Assessment
Pre-Activity Assessment
Pre-Quiz: Students will answer a multiple choice quiz about thermal energy, light refraction, and particle sizes in the Pre/Post-Quiz. They will also answer six questions about the same concepts as well as liquid crystals. Use this assessment prior to any pre-teaching or activities and have them compare their responses after the activity to see their growth and new understanding. Use the Pre/Post-Quiz Answer Key for grading.
Activity Embedded Assessment
Data collection and lab report: Use the questions in the Liquid Crystal Lab Student Sheet to determine student understanding of the topic.
Post-Activity Assessment
Post-Quiz: Re-conduct the Pre/Post-Quiz to gauge student understanding. Use the Pre/Post-Quiz Answer Key for grading.
Investigating Questions
- What consistency do the liquid crystals remind you of? (Example: it is similar to honey.)
- LCD’s are in computers, TVs etc. What do you think would happen if you applied a lot of heat to one of these LCD screens? (Example: I think that the screen would change colors.)
- What color is observed at the hottest point? (Red) At the coolest point? (Blue)
- What is the sequence of colors observed? (Blue, green, orange/yellow, red)
- Are the colors more vivid on a white or black background? (Black)
Safety Issues
- Chemicals should not be inhaled and should be used under adult supervision at all times.
- Protective eyewear and gloves are necessary. When materials are hot, or hot plate/heat gun are being used, take appropriate protective measures such as wearing heat resistant gloves.
Troubleshooting Tips
For reference the following quantities make the various temperature ranges:
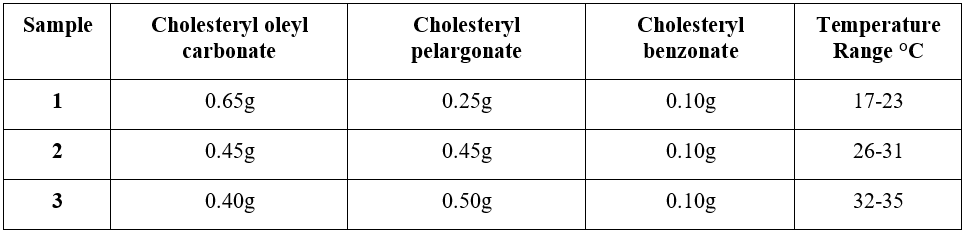
If the liquid crystal is solidifying too quickly:
- Have the students transfer the liquid crystal onto the contact paper directly from the hot plate (teacher supervision for this is necessary).
- Make sure the materials are completely melted.
Activity Extensions
* Students can create various crystals including salt crystals and change the varying concentrations etc.
Activity Scaling
- For lower grades, the teacher can demonstrate the creation of the liquid crystals, but use pre-made liquid crystal sheets for the experimentation. Or the liquid crystal synthesis can be done as a whole class activity.
- For higher grades, students can research the structure of liquid crystals and determine what is changing that causes the various wavelengths to be reflected. They can create a helix model out of Popsicle sticks to demonstrate this as well.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the difference between temperature and thermal energy. They create thermometers using simple materials and develop their own scales for measuring temperature. They compare their thermometers to a commercial thermometer, and get a sense for why engineers need to understand the pr...
References
Chen, S.-H., and Chen W.-J., “Simulation on the Kinetic Process in Polymer-Dispersed Liquid Crystals: Effects of Various Concentration of Liquid Crystals.” Molecular Crystals and Liquid Crystals Science and Technology. Section A. Molecular Crystals and Liquid Crystals. 1997, 363–370. doi:10.1080/10587259708042015.
Gray, G. W. Thermotropic Liquid Crystals. Published on Behalf of the Society of Chemical Industry by John Wiley & Sons, 1987
Helmenstine, Anne Marie. “How to Make Homemade Rock Candy or Sugar Crystals.” ThoughtCo, ThoughtCo, www.thoughtco.com/how-to-grow-sugar-crystals-607659.
“Liquid Crystals Lab.” Chemical Engineering UC Davis, University of California Davis, che.engineering.ucdavis.edu/wp-content/uploads/2014/06/GAANN-exp-5-Liquid-Crystal-Lab.pdf.
Ravnik, Miha, and Slobodan Žumer. “Nematic and Chiral Nematic Liquid Crystals in Confined Geometries: Elementary Background with Selected Examples.” Handbook of Liquid Crystals, 2014, pp. 1–22., doi:10.1002/9783527671403.hlc092
Tyson, Jeff. “How LCDs Work.” HowStuffWorks, HowStuffWorks, 17 July 2000, electronics.howstuffworks.com/lcd1.htm.
Copyright
© 2020 by Regents of the University of Colorado; original © 2019 University of TexasContributors
Amanda Lengnick-HallSupporting Program
NASCENT (Nanomanufacturing Systems for Mobile Computing and Mobile Energy Technologies) Engineering Research Center - Research Experiences for Teachers Program, University of Texas at AustinAcknowledgements
This material is based upon work supported by the National Science Foundation under grant no. EEC-1160494—a Research Experience for Teachers program titled “Nanomanufacturing Systems for Mobile Computing and Mobile Energy Technologies, or NASCENT.” Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Last modified: February 26, 2020



User Comments & Tips