
Summary
This lesson is meant to introduce students to corrosion and engineering techniques that help prevent corrosion. To launch the lesson, students are given a few samples of nails for a mini-activity. Different types of nails are placed in multiple water solutions (salt water vs. tap water) over specified periods of time (2 weeks, 1 week, etc.) so that students can see a variety of outcomes. Without being told about those conditions, students have a discussion in small groups to record their observations, explanations, and questions. Students learn about the negative impact that corrosion can have on human-made structures such as bridges, boats, and cars. They read an article and watch a short video to learn about what corrosion is and why it occurs. During a class discussion with guided notes, students are given examples and share their own experiences of how corrosion affects multiple areas of everyday life. They also discuss how and why industries and individuals take steps to prevent corrosion from occurring.Engineering Connection
Corrosion has an adverse effect on many industries by lowering the shelf life of various metal products. With further research and intentional planning, engineers can design solutions to prevent corrosion to prolong the life of different metals and their applications. Additionally, while we know why corrosion occurs, engineers must apply their understanding of this process to find the most cost-effective methods to prevent corrosion.
Learning Objectives
After this lesson, students should be able to:
- Explain corrosion in their own words.
- Explain why some metals rust or oxidize.
- Provide examples of corrosion and corrosion prevention in everyday life.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-1. Analyze a major global challenge to specify qualitative and quantitative criteria and constraints for solutions that account for societal needs and wants. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze complex real-world problems by specifying criteria and constraints for successful solutions. Alignment agreement: | Criteria and constraints also include satisfying any requirements set by society, such as taking issues of risk mitigation into account, and they should be quantified to the extent possible and stated in such a way that one can tell if a given design meets them. Alignment agreement: Humanity faces major global challenges today, such as the need for supplies of clean water and food or for energy sources that minimize pollution, which can be addressed through engineering. These global challenges also may have manifestations in local communities.Alignment agreement: | New technologies can have deep impacts on society and the environment, including some that were not anticipated. Analysis of costs and benefits is a critical aspect of decisions about technology. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-2. Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students' own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future. Alignment agreement: | The periodic table orders elements horizontally by the number of protons in the atom's nucleus and places those with similar chemical properties in columns. The repeating patterns of this table reflect patterns of outer electron states. Alignment agreement: The fact that atoms are conserved, together with knowledge of the chemical properties of the elements involved, can be used to describe and predict chemical reactions.Alignment agreement: | Different patterns may be observed at each of the scales at which a system is studied and can provide evidence for causality in explanations of phenomena. Alignment agreement: |
Common Core State Standards - English
-
Determine the central ideas or conclusions of a text; summarize complex concepts, processes, or information presented in a text by paraphrasing them in simpler but still accurate terms.
(Grades
11 -
12)
More Details
Do you agree with this alignment?
-
Integrate and evaluate multiple sources of information presented in diverse formats and media (e.g., quantitative data, video, multimedia) in order to address a question or solve a problem.
(Grades
11 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the characteristics and scope of technology.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Michigan - Science
-
Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Analyze a major global challenge to specify qualitative and quantitative criteria and constraints for solutions that account for societal needs and wants.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/mis-2476-corrosion-crisis-prevent-lesson] to print or download.Pre-Req Knowledge
- Prior to this lesson, students should understand the difference between chemical and physical changes, and provide examples for both. Students should also be able to recognize signs that a chemical reaction has taken place.
- Students should know and have their own understanding of the following vocabulary terms:
- chemical change/reaction
- product
- reactant
Introduction/Motivation
- Have the first slide of the How Can We Prevent the Corrosion Crisis? Presentation open and visible to students at the start of class.
- The printout of notes, How Can We Prevent the Corrosion Crisis? Notes, should be in a pile near the nail samples, one for each student.
- Warm-Up Mini-Activity (see below for how to prep for this section)
- Individual:
- A few weeks or days prior to the lesson, submerge a handful of different types of nails in a couple types of water (tap, salt, etc.) On the day of the lesson, display the samples at the front of the room in labeled test tubes or in jars. Students should not know what type of nail is being used, what type of water it is placed in, or how long it’s been placed in the water. As students come into class, the directions will be presented on the board (as part of the slides). Students will be asked to record their observations, explanations, and questions about the nail samples at the top of their notes.
- Turn, Talk, and Share
- Teacher will have students work in small teams (no more than four students). Students will take turns discussing what they wrote. After a few minutes, the teacher will lead the class in a discussion, focusing on some of the following:
- Trial setups (use of water, type of nail, time submerged)
- Student observations (amount of rust or observable corrosion)
- Factors as to why a nail did or did not rust (or the severity of the rust)
- KWLS Chart
- “You’ve seen some examples of nails, some that have corroded and some that have not. Before we start the lesson, you are going to record what you already know about corrosion.” Students will fill out the K in their KWLS chart.
- “Now, think about what you want to know about corrosion. Record one or two thoughts or questions in your W bubble.” Students will fill out the W in their KWLS chart.
- Lesson Introduction
- “During this lesson, we are going to discuss what corrosion is and why it occurs. We will briefly discuss examples of corrosion in everyday life and how different industries and individuals are trying to prevent corrosion.”
- Describes the goals of the lesson and what will be discussed.
- Follow How Can We Prevent the Corrosion Crisis? Presentation (see notes for what to say or discussion points)
- Cost of corrosion (2 slides)
- Article
- Assign students the short article called Corrosion via Google Drive. This article can be printed or viewed online. With both methods, students can highlight and make notes as they read.
- Video
- As a class, watch “Rust : Prevention & Treatment | Environmental Chemistry | Chemistry | FuseSchool” on YouTube: https://www.youtube.com/watch?v=jQoE_9x37mQ.
- KWLS Chart (continued)
- “Now that we’ve watched a video, read an article, and have been given some background about corrosion, what is something new that you’ve learned about corrosion? Write two or three comments in your L bubble.”
- Guided notes & class discussion:
- What is corrosion? What is rust? Why do they occur?
- Examples of corrosion in everyday life (student led)
- How can corrosion be prevented?
- KWLS Chart (finish)
- “Now that we’re at the end of the lesson, I want you to write down one or two things that you still want to know about corrosion. This should be done individually on your notes.”
- Sticky Note Activity
- In pairs or small groups, students share what they wrote for S.
- Each group will be given one or two sticky notes to write their question or thought on. (One idea per sticky note).
- Place sticky notes at the front of the classroom on the whiteboard. If there is time, read each sticky note to the class. With the group’s help, organize them in categories that are similar.
- These sticky notes can be referred to later in the unit or during the activity.
Lesson Background and Concepts for Teachers
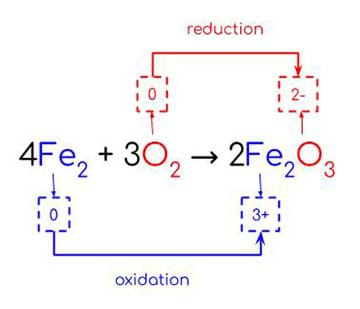
Corrosion is the deterioration of metals due to redox reactions. Oxidation-reduction reactions, or redox reactions, are chemical reactions that involve a transfer of electrons. To determine whether or not a redox reaction has occurred, oxidation numbers can be compared for the reactants and products.
In a redox reaction, oxidation and reduction occur simultaneously. Oxidation is the loss of electrons or increase in oxidation state. Reduction is the gain of electrons or decrease in oxidation state (Figure 1).
There are two mnemonics to help students remember the difference between oxidation and reduction. “OIL RIG” represents “Oxidation is Losing (electrons), Reduction is Gaining (electrons)”. “LEO says GER” represents “Loss of Electrons = Oxidation, Gain of Electrons = Reduction.”
For more information on Oxidation-Reduction Reactions & Oxidation States:
- LibreTexts – Chemistry: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
- Lumen – Boundless Chemistry: https://courses.lumenlearning.com/boundless-chemistry/chapter/oxidation-reduction-reactions/
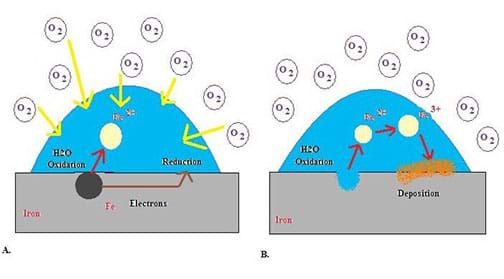
A metal, oxygen, and an electrolyte are the three main components required for corrosion to occur. The metal, which is being corroded, is oxidized and forms free electrons. The oxygen is then reduced by the free electrons.
The dissolution of the metal at the anode (where oxidation takes place) causes the metal ions to become hydrated in the solution. If this occurs, further oxidation of the metal ions can occur, forming an open pit. The dissolution of the metal could also cause the metal ions to form a solid compound that collects on the surface of the metal. A protective barrier may form and inhibit further corrosion.
For more information about corrosion and how it occurs:
The impact of corrosion is not the same for all metals. Some metals do not corrode due to their chemical properties. Other metals form a protective layer on the outside when oxidized, preventing further corrosion from occurring. The type of corrosion that most people are familiar with is rust (iron oxide), which can deteriorate the metal and cause it to become flaky. With enough time, the corrosion can wear down the metal, causing it to fall apart.
Corrosion has a lot of adverse impacts on the environment and society. If infrastructure is not monitored or maintained, structures can collapse or crumble. Water pipes and storage tanks can become corroded, releasing harmful metals and bacteria into the water.
In addition to health and safety concerns, corrosion also has a large financial cost. Metal structures that become corroded need to be repaired or replaced overtime. If not treated initially, more resources may be required to continually repair and maintain structures.
Impacts of Corrosion Resources:
- What does corrosion cost? (Thomas Industrial Coatings): https://www.thomasindcoatings.com/what-does-corrosion-cost/
- Flint Water Crisis: https://www.iflscience.com/editors-blog/science-behind-flint-water-crisis-corrosion-pipes-erosion-trust/
- Economic Impact of Corrosion: http://impact.nace.org/economic-impact.aspx
- Cost of Corrosion (G2MT Laboratories): https://www.g2mtlabs.com/cost-of-corrosion/#:~:text=The%20total%20annual%20corrosion%20costs,at%20%241.1%20trillion%20for%202016
- Corrosion Cost to Society (1998 study): https://corrosion-doctors.org/Principles/Cost.htm
Because of the negative impact that corrosion has, engineers use multiple techniques to prevent corrosion of metals. A few examples of this are coating, sacrificial coatings, and cathodic protection.
Preventing Corrosion: https://courses.lumenlearning.com/introchem/chapter/preventing-corrosion/
Associated Activities
- Keep Your Boat Afloat - Students engineer a ship that not only holds cargo but also resists corrosion. Students choose the design and shape of ship, the metal used to make the ship, and the type of coating that will prevent corrosion from occurring. After the initial design and build, students “set their ships to sea” and then monitor their ship daily, collecting observations.
Lesson Closure
Introduce the Keep Your Boat Afloat activity by briefly discussing cargo ships and preventing corrosion. Challenge the students to engineer a ship that can hold cargo and resist corrosion.
Vocabulary/Definitions
corrosion: Chemical change of a metal surface when it’s exposed to oxygen in the environment.
metal: A type of matter that is a good conductor of heat and electricity. Properties: typically hard, lustrous, ductile, malleable, and fusible.
rate of reaction: How fast a chemical reaction takes place; in other words, how fast a product is formed or a reactant is used up.
rust: Corrosion of iron and its alloys. A reddish- or yellowish-brown flaking coating of iron oxide.
surface area: The amount of reactant that is exposed to other reactants in a chemical reaction. The greater the surface area of a solid reactant, the faster its rate of reaction.
Assessment
Pre-Lesson Assessment
Turn & Talks/Discussion Questions: Students share their observations from the nail samples in small groups (prompts provided on PowerPoint slide 3).
Lesson Summary Assessment
KWLS Chart & Notes: As students work through the lesson, they record complete a KWLS chart and write notes on individual note sheets.
Sticky Note Exit Ticket: In small groups, students use sticky notes to share what they still want to know about corrosion (prompts provided on PowerPoint slide 24).
Lesson Extension Activities
If time allows, teachers could use this activity to introduce redox reactions, but go into more detail. After this lesson (and associated activity), Teachers can teach the rate of reactions in more detail as well as different types of reactions.
Teachers could have students research different types of coatings/treatments used in multiple industries for the treatment or prevention of corrosion.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about nondestructive testing, the use of the finite element method (systems of equations) and real-world impacts, and then conduct mini-activities to apply Maxwell’s equations, generate currents, create magnetic fields and solve a system of equations. They see the value of NDE and FEM...
References
(See links referenced in lesson background and concepts for teachers)
Copyright
© 2020 by Regents of the University of Colorado; original © 2019 Michigan State UniversityContributors
Jennifer RothSupporting Program
RET Program, College of Engineering, Michigan State UniversityAcknowledgements
This lesson was created due to funding from the National Science Foundation and Research Experience for Teachers program at Michigan State University in East Lansing, Michigan. NSF Site Research Experience for Teachers, College of Engineering, Michigan State University RET Grant#1609339.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation.
Last modified: July 20, 2023



User Comments & Tips