Quick Look
Grade Level: 11 (9-12)
Time Required: 30 minutes
Lesson Dependency:
Subject Areas: Biology, Chemistry, Life Science, Science and Technology
NGSS Performance Expectations:

| HS-ETS1-1 |

Summary
Students learn about various crystals, such as kidney stones, within the human body. They also learn about how crystals grow and ways to inhibit their growth. They also learn how researchers such as chemical engineers design drugs with the intent to inhibit crystal growth for medical treatment purposes and the factors they face when attempting to implement their designs. A day before presenting this lesson to students, conduct the associated activity, Rock Candy Your Body.Engineering Connection
Numerous kinds of crystals occur in the human body. Many of these crystals impair humans by causing pain or disabilities. Engineers and physicians study the mechanisms of crystal growth to determine ways in which crystal growth can be blocked. They design new drugs that attach to crystals to inhibit further growth, are biocompatible with minimal side effects to the human body, and can be administered efficiently and effectively.
Learning Objectives
After this lesson, students should be able to:
- Describe the process of crystallization in the body and how it affects the normal functions of the urinary system.
- List other crystallization processes in the human body and the problems they may cause.
- Identify how engineers design drugs and why they choose certain ones over others.
- Explain efficacy and potency and the differences between the two.
- Describe why crystals may form different shapes when inhibition occurs.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-1. Analyze a major global challenge to specify qualitative and quantitative criteria and constraints for solutions that account for societal needs and wants. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze complex real-world problems by specifying criteria and constraints for successful solutions. Alignment agreement: | Criteria and constraints also include satisfying any requirements set by society, such as taking issues of risk mitigation into account, and they should be quantified to the extent possible and stated in such a way that one can tell if a given design meets them. Alignment agreement: Humanity faces major global challenges today, such as the need for supplies of clean water and food or for energy sources that minimize pollution, which can be addressed through engineering. These global challenges also may have manifestations in local communities.Alignment agreement: | New technologies can have deep impacts on society and the environment, including some that were not anticipated. Analysis of costs and benefits is a critical aspect of decisions about technology. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of and be able to select and use medical technologies.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
evaluate models according to their limitations in representing biological objects or events; and
(Grades
9 -
11)
More Details
Do you agree with this alignment?
-
in all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student;
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/uoh_crystals_lesson01] to print or download.Pre-Req Knowledge
A basic understanding of the urinary system and how it functions, as well as a basic chemistry background, specifically an understanding of solubility and pH. Also useful know about the digestive system for a discussion on how drugs can be administered.
Introduction/Motivation
(First, conduct the associated activity, Rock Candy Your Body: Exploring Crystallization, the day before presenting this lesson to students. Then, be ready to show students the 13-slide Crystallization in the Body Presentation, a Microsoft PowerPoint® file, to teach the lesson. In advance, make copies of the Crystallization Worksheet, one per student. Begin by asking the pre-assessment questions as a class discussion and then presenting the Introduction/Motivation content. Continue by showing the class the presentation and delivering the content in the Lesson Background section to teach them about the crystallization process and why it is important.)
Throughout the human body, crystals can form, sometimes causing pain or disabilities. In the urinary system, waste is removed by the kidneys and transported to the bladder to be excreted. However, millions of people develop kidney stones, which block the removal of the waste, often painfully. How do these large stones form? How are they removed? What treatments are available? Kidney stones form through a crystallization process. Researchers, including chemical engineers, investigate and experiment to design drug inhibitors that bind to crystal faces so as to block further growth, thus reducing the number of kidney stones people pass.
Another example of crystals in the human body can be seen in malaria. The parasite causing the disease eats human red blood cells, breaking hemoglobin into hematin. Hematin is toxic to the parasite. To prevent death, the parasite sequesters the hematin into crystals. Current anti-malarial drugs work by inhibiting the crystallization of hematin, resulting in the parasite's death.
Each crystal has a different shape and structure that is unique. What molecules are able to bind to each crystal? What makes one molecule more effective than another? Researchers, including chemical and biomedical engineers, examine a variety of factors to determine what molecules may or may not be effective in blocking crystal growth.
Lesson Background and Concepts for Teachers
(slide 1) In the body, crystals are made in various systems. However, do you know: Where are crystals made? How are crystals formed? Why do our bodies perform this process?
(slide 2) What are examples of crystals found in nature? (Pictures show rock salt, diamonds, snowflakes.) Salt is a crystal, including the salt you put on your food. Most gemstones are crystals and form over long periods of time. Snowflakes are ice crystals that form unique shapes based on the conditions that exist when they fall to the ground.
(slide 3) What are examples of crystals found in the body? (Pictures show aspirin, hemozoin found in malaria, cataracts and insulin.) Aspirin is a commonly used painkiller. Most drugs are in crystalline forms. The parasite that causes malaria eats red blood cells, which breaks down the hemoglobin into hematin, which is toxic to the parasite. The parasite saves itself by crystallizing hematin into hemozoin. Cataracts are caused by the crystallization of eye proteins, causing the eye to become opaque. Proteins in the eye do not replenish themselves.
(slide 4) What is a crystal? A crystal is a solid material whose atoms, molecules or ions are arranged in an ordered pattern extending in all directions. Crystals form a variety of shapes depending on how the molecules pack together. These diagrams illustrate the variety of shapes.
(slide 5) Crystal Surface and Growth Process. Each side of a crystal is called a face. During crystal growth, steps are basically layers or sheets of molecules stacked on top of each other. A terrace is the flat surface between steps. Crystal surfaces have five places where molecules can bind: 1) The first is directly to the surface with no molecules surrounding it. This position permits one bond to be made. 2) The second position is on a terrace next to a step where two bonds can be made. 3) The third position is on a terrace in the corner of a step where three bonds can be made. This is called a kink position. 4) The fourth position is a hole in a step on a terrace where four bonds can be formed. 5) The fifth position is a hole in the terrace where five bonds are possible. Of all these possible positions, a kink is the most likely place where molecules attach to the crystal surface and continue to grow. The shape of a crystal is determined by the speed of step growth on each face of the crystal.
(slide 6) Crystallization. During the process of crystallization, three phases occur: During the first phase, clustering, molecules agglomerate together. During the second phase, nucleation, the clusters of molecules become a stable nuclei and arrange themselves into an ordered pattern, which is the basic building structure of the crystal. If the clusters are unstable, the molecules re-dissolve and begin the clustering phase again. If nucleation occurs, then the third growth phase begins; at this point, more molecules attach to the stable nuclei, so the crystal grows in size. The driving force of this process is supersaturation. Supersaturation occurs when more solute exists than the solvent can dissolve. A crystal continues to grow until the solution is saturated. Crystal nucleation and growth can occur simultaneously, depending on the conditions of the system. Usually, one or the other is predominant, causing different crystal sizes and shapes. Polymorphism occurs when one compound can crystallize with different crystal structures.
(slide 7) Making rock candy is a good example of the crystallization process. Sugar is dissolved in water, with more sugar dissolving at higher temperatures. Supersaturation occurs when the solution is left at room temperature, due to differences in solubility. The higher the supersaturation, the faster the growth. Seed crystals cause the growth to start immediately and skip past the nucleation phase.
(slides 8-9) Growth Inhibition and Shape Changes. To inhibit growth, drug molecules are designed to bind to the crystal surface. Most drug molecules incorporate into the kinks on the surface, blocking further growth. However, sometimes binding to the kink site has no effect on stopping crystal growth; the crystal may grow around the bound drug molecule. In these cases, more than one drug molecule may be required to block crystal growth. As an analogy, think of a stream with rocks in it. If one large rock is in the stream, the water flows around it. Multiple rocks or a dam are needed in order to block water flow entirely. Drug molecules usually only bind to a specific face, which inhibits growth in one direction; this causes the crystal to have a different shape, or morphology, since growth continues in the other directions, as shown in Figure 1.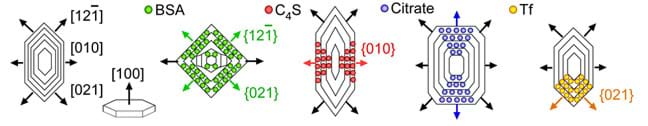
(slides 10-11) Kidney Stones. The kidneys filter the blood, remove waste products, and regulate electrolytes and blood pressure. Dehydration is one contributor to the formation of kidney stones. When the body is dehydrated, little water is removed by the kidneys, resulting in a supersaturation of minerals, a precursor to the growth of kidney stones. Within the kidney, four main types of kidney stones can occur. Calcium-containing stones are the most common, usually in the form of calcium oxalate. Oxalate is made by the liver and is also found in many foods such as nuts, chocolate, fruits and vegetables. Struvite stones are formed in response to an infection. Uric acid stones occur when the body does not intake enough fluid or too much protein. Cystine stones form in those who have certain hereditary disorders in which the kidney releases too much amino acid.
(slides 12-13) Drug Design. When designing new drugs, a variety of factors must be considered: efficacy, potency, toxicity, administration and cost. By efficacy we mean how well a drug is able to inhibit crystal formation. For example, causing 100% inhibition is a high efficacy. The potency of the drug is determined by how much of the drug is necessary to produce a large amount of inhibition. A small amount of drug that produces a high-percentage of inhibition means that the drug is potent. The toxicity of the drug also must be considered. How many and what types of side effects occur when introduced into the body? For example, mercury might be a potent, high-efficacy drug, but it is extremely toxic to the human body, so it is not usable. How might the drug be administered or put into the human body? Some delivery methods: oral (through the digestive tract), rectal, aerosol or intravenous. The physical properties of a drug or encapsulation of a drug must be tailored to suit the administration method of choice. What is the cost to make the drug? If manufacturing of the drug is not cost-effective, it cannot be mass-produced in order to be made available to the public.
Associated Activities
- Rock Candy Your Body: Exploring Crystallization - While making rock candy, students determine how sugar crystals are affected when different additives are incorporated into a supersaturation solution. They predict which will be more effective based on their properties and contents. Begin this activity one day before the lesson is taught to students.
- Kidney Stone Crystallization - Student teams examine how calcium oxalate crystals are affected by inhibitors. Like chemical engineers, they determine which inhibitor would be the best for blocking crystallization.
Lesson Closure
For centuries, people have been taking human-made herbal concoctions and drugs as remedies for body afflictions. During the 19th century, pharmaceutical companies came into existence and now represent a $1 trillion industry. New drugs are continuously in development, being specifically designed to address incurable diseases, disease prevention, and improvement of current remedies and treatments.
Vocabulary/Definitions
calcium oxalate: A molecule that comprises the most common kidney stone.
clustering: The first phase of the crystal-forming process during which molecules agglomerate.
crystal: A solid material that consists of an ordered pattern in all directions.
cystine: An amino acid that is overproduced due to a hereditary disorder, causing kidney stones.
dehydration: The excess loss of body water.
efficacy: A measure of how much a drug is able to inhibit. For example, causing 100% inhibition is high efficacy.
face: A side of a crystal.
growth: The third phase of crystallization during which more molecules attach to the stable nuclei, causing the crystal to grow in size.
inhibition: The disruption of normal crystal growth, or blockage of further growth.
kidney: An organ in the urinary system that removes waste products, and regulates electrolyte levels and blood pressure.
kidney stone: A crystalline structure that forms in the kidney due to supersaturation, causing blockages to waste removal.
kink: A position on a crystal surface where molecules are most likely to attach.
nucleation: The second phase of crystallization during which clusters of molecules become a stable nuclei. The molecules arrange themselves into an ordered pattern, which is the basic building structure of the crystal.
potency: A measure of how much of a drug produces a large amount of inhibition.
solubility: The maximum amount of solute that can be dissolved into a solvent.
solute: In a solution, the component that is being dissolved into another.
solvent: In a solution, the component that another component is dissolved into.
step: In a crystal, a layer or sheet of molecules stacked on top of each other.
struvite: A phosphate mineral that forms one type of kidney stone.
supersaturation: A condition in which the amount of solute dissolved in a solvent is above the solubility.
terrace: On a crystal face, the flat surface between steps.
toxicity: A measure of the degree of harmfulness of a molecule to humans.
uric acid: A product of nucleotide breakdown in the body that causes kidney stones.
urinary system: The organ system that removes waste from the body and regulates electrolytes, blood pressure and blood pH.
Assessment
Pre-Lesson Assessment
Discussion Questions: Ask students to name crystals that can be found in nature. Do they form different shapes from each other? Talk about snowflakes, which are ice crystals; each snowflake forms a different unique shape due to the conditions it faces when falling to the ground. Ask students if they take ibuprofen or aspirin; these are crystals. What are these drugs? Almost every drug is a crystal.
Lesson Summary Assessment
Worksheet: Have students complete the six-question Crystallization Worksheet on kidney stones, crystallization, inhibition and drug design. Review their answers to assess their mastery of the subject matter.
Lesson Extension Activities
Have students use microscopes to look at common crystals such as salt, aspirin (remove any pill coating) and graphite (pencil lead). Expect them to notice that the crystals have faceted edges, which means they are highly defined and sharp looking. Depending on how the crystals are oriented under the microscope, students may be able to see different faces, defects, and how certain steps (layers) continue to grow and look like small towers on the crystal face. Watch time lapse videos of crystal growth; see website suggestions in the Additional Multimedia Support section. See examples of salt and sugar under the microscope at http://montessorimuddle.org/2011/04/24/salt-and-sugar-under-the-microscope/.
Additional Multimedia Support
Timelapse Macro Video of Crystal Growth (1 minute; dendrite branching): https://www.youtube.com/watch?v=K297toCvHtY
Crystal Growth Time Lapse through microscope (55 seconds): https://www.youtube.com/watch?v=7vb84ShuiJw
KDP Crystal Growth (55 seconds; giant growth for industrial application): https://www.youtube.com/watch?v=l_USYub3djY
Animation of growth inhibitor attacking active spiral growth sites (~4 seconds, repeating): http://baso4scaling.wikispaces.com/Crystal+Growth
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn how crystallization and inhibition occur by examining calcium oxalate crystals with and without inhibitors that are capable of altering crystallization. Students play the role of engineers by trying to determine which inhibitor would be the best for blocking crystallization.

Students see and learn how crystallization and inhibition occur by making sugar crystals with and without additives in a supersaturation solution, testing to see how the additives may alter crystallization, such as by improving crystal growth by more or larger crystals.

Students are challenged to think as biomedical engineers and brainstorm ways to administer medication to a patient who is unable to swallow. They learn about the advantages and disadvantages of current drug delivery methods—oral, injection, topical, inhalation and suppository—and pharmaceutical desi...
References
Chernov, A. A. Modern Crystallography III, Crystal Growth. Berlin, Germany: Springer, 1984. http://www.springer.com/materials/surfaces+interfaces/book/978-3-642-81837-0
Farmanesh, Sahar, Sriram Ramamoorthy, et al. (2013) "Specificity of Growth Inhibitors and Their Cooperative Effects in Calcium Oxalate Monohydrate Crystallization." Journal of the American Chemical Society. Vol. 136, No. 1, pp. 367-376. http://pubs.acs.org/doi/abs/10.1021/ja410623q
Copyright
© 2014 by Regents of the University of Colorado; original © 2014 University of HoustonContributors
Andrea Lee, Megan KetchumSupporting Program
National Science Foundation GK-12 and Research Experience for Teachers (RET) Programs, University of HoustonAcknowledgements
This digital library content was developed by the University of Houston's College of Engineering, based upon work supported by the National Science Foundation under GK-12 grant no. DGE 0840889. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Last modified: January 31, 2018




User Comments & Tips