Quick Look
Grade Level: 10 (9-10)
Time Required: 15 minutes
Lesson Dependency: None
Subject Areas: Chemistry, Physics

Summary
Students are introduced to statics and dynamics, free-body diagrams, combustion and thermodynamics to gain an understanding of the forces needed to lift rockets off the ground. They learn that thrust force is needed to launch rockets into space and the energy for thrust is stored as chemical energy in the rocket's fuel. Then, using the law of conservation of energy, students learn that the chemical energy of the fuel is converted into work and heat energy during a rocket launch. A short PowerPoint® presentation is provided, including two example problems for stoichiometry review. An optional teacher demonstration is described as an extension activity.Engineering Connection
Rockets are an engineering marvel. Mechanical and aerospace engineers develop the shape and design of the rocket, chemical engineers design the fuel, and materials engineers develop lightweight, thermally durable materials for trips into outer space. This lesson serves as preparation to conduct the associated activity during which students use their knowledge of stoichiometry to create the fuel for a small rocket engine as well as their critical thinking skills to design a rocket that can be successfully launched.
Learning Objectives
After this lesson, students should be able to:
- Draw a free-body diagram.
- Apply Newton's second law of motion.
- Describe the effect of forces on motion.
- Identify the components needed for a combustion reaction.
- Explain the law of conservation of energy.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
Common Core State Standards - Math
-
Define appropriate quantities for the purpose of descriptive modeling.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
In order to select, use, and understand energy and power technologies, students should learn that:
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
Science concepts. The student recognizes multiple forms of energy and knows the impact of energy transfer and energy conservation in everyday life. The student is expected to:
(Grades
9 -
10)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/uoh_liftoff_lesson01] to print or download.Pre-Req Knowledge
Students should be able to balance chemical equations using stoichiometry.
Introduction/Motivation
How do rockets work? Rockets are large and extremely heavy, and therefore require a great deal of energy to get them off of the ground. But from where does this energy come? The chemical energy stored in the fuel of the rocket is transformed into heat and work. Heat energy is released during the combustion of the fuel and the work energy is evident by the rocket's ability to launch itself off the ground. This lesson is designed to give you a basic understanding of the physics and chemistry of launching rockets.
Lesson Background and Concepts for Teachers
Show students the six-slide Rockets Presentation, a PowerPoint® file, as you provide the information below.
(slide 1) Imagine a person that is standing still; the weight of the person acting downward onto the ground must be equal to the normal force of the ground acting upward on the person. Draw for students a free body diagram to depict this concept, such as the free body diagrams shown in Figures 1 and 2 and on slide 1. 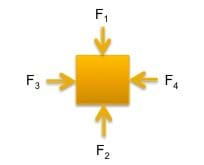
In order for the object to remain stationary, the magnitudes of the forces must be equal, F1 = F2 and F3 = F4. Or if we use vectors, which include magnitude and direction, F1 = -F2 and F3 = -F4. Another way to mathematically describe this is to say that the sum of the forces is equal to zero, ΣF = 0.
In order to move the object in an upper-left diagonal motion (see Figure 2), then F2 > F1, and F4 > F3 must be true. Therefore, ΣF ≠ 0.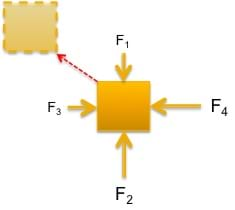
(slide 2) To apply this to a rocket, we must identify the forces acting on the rocket. Based on Newton's second law of motion, we know that F = ma (force is equal to the mass of an object multiplied by its acceleration). Thus, the weight of any object on Earth is equal to its mass multiplied by the gravitational acceleration constant of 9.81 m/s2. In order to lift a rocket off the ground, a force must act upward on the rocket with a larger magnitude than the rocket's weight (see Figure 3). This is the thrust force.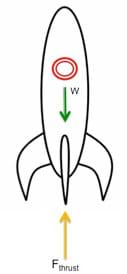
The equation for thrust is Fthrust = uR, where u is the exhaust velocity and R is the rate of mass ejection or the change in the amount of fuel over time.
(slides 3-5) From where does the energy for thrust come? Now, we apply the first law of thermodynamics, the conservation of energy (ΔE=ΔQ+ΔW). The energy needed for thrust comes from the chemical energy stored in the fuel. You determine the fuel's chemical potential by finding its Gibbs energy. Because energy cannot be created or destroyed, the chemical energy of the fuel is converted into heat energy and work energy. The heat energy is from the combustion of the fuel (breaking of the chemical bonds) and the work energy corresponds to how high the rocket flies. The three components needed for a combustion reaction are oxygen, fuel and a heat source (see Figure 4).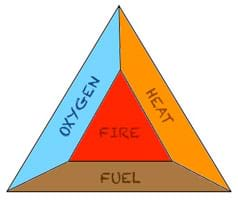
(slide 6) A prerequisite for the associated activity (Refer to We Have Liftoff) is knowledge of stoichiometry. This slide provides a brief stoichiometry review with two example problems. The first problem asks students to determine how many grams of water are formed in the combustion reaction of 85.0 grams of propane in excess oxygen. The second example asks students to determine how many moles of hydrogen are produced in the reaction of 20.0 grams of iron oxide and superheated steam. The solutions include reaction equations and the calculations, with color-coordinated hashes that show the unit cancellations.
Associated Activities
- We Have Liftoff - Students design their own rocket engines using sugar and potassium nitrate as fuel. They are given the unbalanced, combustion-reaction equation of the fuel and must use their knowledge of stoichiometry to determine the correct amount of potassium nitrate that needs to be added to 6 grams of sugar. Once the engines are completed, students launch their rocket engines to witness the success or failure of their designs.
Vocabulary/Definitions
conservation of energy: Energy can neither be created nor destroyed, but can change form.
dynamics: The branch of mechanics concerned with the motion of bodies under the action of forces.
statics: The branch of mechanics that is concerned with the analysis of loads on physical systems in static equilibrium, that is, in a state in which the relative positions of subsystems do not vary over time, or in which components and structures are at a constant velocity.
Assessment
Pre-Lesson Assessment
Discussion Questions: Before beginning the lesson, ask students the following questions to gauge their base knowledge of the lesson topics:
- What is happening if an object is static? (Answer: The forces acting on the object are equal in magnitude. Refer to Figure 1.)
- What is happening if an object is dynamic? (Answer: The forces acting on the object are not equal in magnitude, meaning that one or more forces are greater than the others. This imbalance of forces results in movement of the object. Refer to Figure 2.)
- What does conservation of energy mean? (Answer: Energy can neither be created nor destroyed, but it can change form. In the case of rocket fuel, chemical energy changes to heat energy and work energy.)
Post-Introduction Assessment
Discussion Questions: Ask students the following questions:
- In order for a rocket to launch, what must happen with respect to the forces involved? (Answer: The thrust force must be greater than the rocket's weight.)
- What components are required for a combustion reaction to occur? (Answer: Fuel, oxygen, and heat.)
Lesson Summary Assessment
Discussion Questions: After the presentation, ask students the following questions to check on their comprehension of the lesson content:
- From where does the energy for launching a rocket come? (Answer: The energy for launching a rocket comes from the chemical energy stored in the rocket fuel.)
- What happens to this energy? (Answer: This energy changes to heat and work energies during the combustion-reaction.)
Lesson Extension Activities
As a class demonstration, create your own combustion reaction using a flame as a heat source (such as a Bunsen burner or candle), air for oxygen, and any powder (such as sugar or creamer) as fuel. Prior to the demo, ask the class: What would happen if you put a fairly large amount of powder over the flame. (Answer: The flame would go out because no oxygen would be available for the combustion reaction to occur.) What would happen if the powder was sprinkled over the flame instead? Lightly sprinkle any powder over the flame. Expect to see a "sort of" combustion reaction. Ask students: How does the powder particle size affect combustion? (Answer: Finer powder particles can result in a more homogenous mixture of oxygen and fuel, thus creating a larger combustion.) Tie this into a real-world scenario, for example, factories dealing with food processing or any processing in which an accumulation of dust is possible are at risk for explosions. This has happened to several factories, where silos or canisters are not kept clean and dust accumulates and spreads throughout the air. A single spark or an overheated piece of equipment can cause an entire plant to explode. The success of the demonstration described above is dependent on the room environment and state of the powder. High humidity days or a high moisture content trapped in the powder, used as fuel, could cause a failed demonstration. As an additional extension, discuss spectrums and how different molecules burn different colors. Additives can be used in the fuel to make green or blue flames rather red or orange.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

The purpose of this lesson is to teach students how a spacecraft gets from the surface of the Earth to Mars. Students first investigate rockets and how they are able to get us into space. Finally, the nature of an orbit is discussed as well as how orbits enable us to get from planet to planet — spec...

Students acquire a basic understanding of the science and engineering of space travel as well as a brief history of space exploration. They learn about the scientists and engineers who made space travel possible and briefly examine some famous space missions.
Copyright
© 2015 by Regents of the University of Colorado; original © 2014 University of HoustonContributors
Taylor Dizon-KellySupporting Program
National Science Foundation GK-12 and Research Experience for Teachers (RET) Programs, University of HoustonAcknowledgements
This digital library content was developed by the University of Houston's College of Engineering, based upon work supported by the National Science Foundation under GK-12 grant no. DGE 0840889. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Last modified: July 1, 2019




User Comments & Tips