Quick Look
Grade Level: 5 (4-6)
Time Required: 45 minutes
Lesson Dependency: None
Subject Areas: Chemistry, Earth and Space
NGSS Performance Expectations:

| 5-ESS3-1 |

Summary
Students learn about the differences between surface and ground water as well as the differences between streams, rivers and lakes. Then, they learn about dissolved organic matter (DOM) and the role it plays in identifying drinking water sources. Then students are introduced to conventional drinking water treatment processes by developing and implementing their own water filtration system through the associated activity. A student worksheet and answer key are provided.Engineering Connection
Identifying safe and suitable drinking water sources is a common environmental engineering challenge. When identifying potential water sources, engineers must consider the amount of dissolved organic matter (DOM) that is present. DOM can be transformed into various dangerous chemicals when exposed to chlorine that is usually added during the water treatment process. Before water comes out of the tap, it goes through several treatment steps to make sure it is safe for human consumption. Civil, chemical and environmental engineers design these water treatment processes and facilities in cost-effective ways to meet the needs of communities.
Learning Objectives
After this lesson, students should be able to:
- Describe the differences between streams, rivers and lakes.
- Describe the differences between surface and groundwater.
- Describe the conventional drinking water treatment process.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-ESS3-1. Obtain and combine information about ways individual communities use science ideas to protect the Earth's resources and environment. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Obtain and combine information from books and/or other reliable media to explain phenomena or solutions to a design problem. Alignment agreement: | Human activities in agriculture, industry, and everyday life have had major effects on the land, vegetation, streams, ocean, air, and even outer space. But individuals and communities are doing things to help protect Earth's resources and environments. Alignment agreement: | A system can be described in terms of its components and their interactions. Alignment agreement: Science findings are limited to questions that can be answered with empirical evidence.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Identify problems, and propose solutions related to water quality, circulation, and distribution – both locally and worldwide
(Grade
6)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/cub_drink_lesson01] to print or download.Introduction/Motivation
From where does the water you drink come? Sure, it probably comes out of a sink faucet or drinking fountain, but where was it before that? Today we are going to learn about different water sources, and how that water becomes safe to drink before it ever comes out of a tap. All of these topics are extremely important to environmental engineers, and will help you understand the source of your water. Engineers are in charge of finding good water sources (environmental engineers), treating that water to make it safe (chemical engineers), and then getting that water all the way to you (civil engineers). That means, engineers must think about something called water quality, which is a good indicator of how safe water is to drink. Let's get started!
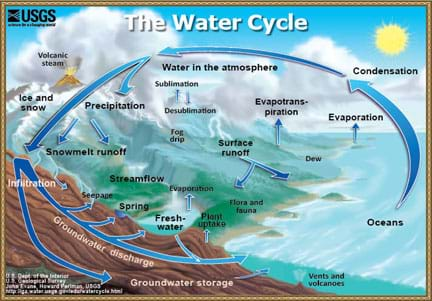
Lesson Background and Concepts for Teachers
Many different types of water exist. The two main water types are surface water and groundwater. Streams, rivers and lakes are all commonly found surface water sources with very important and distinctive differences between them. A stream is a body of water with a current. Streams are visibly moving, and have defined banks, so you can see where the stream ends on either side. A river is a natural waterway (usually freshwater) that flows toward an ocean, lake, sea or another river. The main difference between a stream and river is that a river flows somewhere. A stream can start in a mountain crevice and then flow slowly until it just disappears, but it would be impossible for a river to do so. A lake is generally known as a relatively still body of water—not moving or flowing at a visible rate—that is large in size, and surrounded by land.
Many students are unaware that when they are outside playing on playgrounds or in backyard, water is likely flowing in the ground underneath them! This water is called groundwater. Groundwater is stored in aquifers. One of the most famous aquifers is known as the Ogallala, which is located in the Midwest and used to irrigate crops in that region. In order to water crops with groundwater, engineers must drill wells into the ground and use pumping systems to pull that water to the surface.
Water that is brown in color, often resembling the appearance of a cup of tea, is likely filled with dissolved organic matter, known as DOM. DOM is composed of 50% carbon. Carbon is in practically everything—pencils contain lead that is made of carbon, the human body is about 18% carbon, and trees and soil all contain carbon.
DOM has both positive and negative effects on water. One positive is that microorganisms, little tiny things that can only be seen with a microscope, love DOM! They think it is delicious and you always find more microorganisms when you see water with a lot of DOM. The darker the water, the more food available. On the negative side, DOM has the ability to transport metals. Two of the most common and most dangerous of these metals are mercury and arsenic. When mercury comes into contact with microorganisms and water, it can be transformed into a harmful neurotoxin, meaning it becomes dangerous for the brain. Arsenic often occurs naturally in rock formations and it can be released into groundwater when it comes in contact with DOM. This means that if you see water high in DOM, a good chance exists that it is high in metals. So, environmental engineers must keep this information in mind when choosing safe drinking water sources.
Humans play a role in the amount of DOM found in water. Studies have shown that humans cause 10 times more erosion, the wearing away of the earth's surface, than natural processes [U. of Mich, 2004]. By building houses and roads near streams and rivers, humans can increase DOM content in water. However, humans can help reduce erosion! By planting ground cover on hills and stream banks, roots systems help to maintain the natural structure of the soil, and therefore helps limit erosion.
Many people are unaware of the many steps that water goes through before it comes out of the tap. Figure 1 shows the conventional U.S. drinking water treatment process.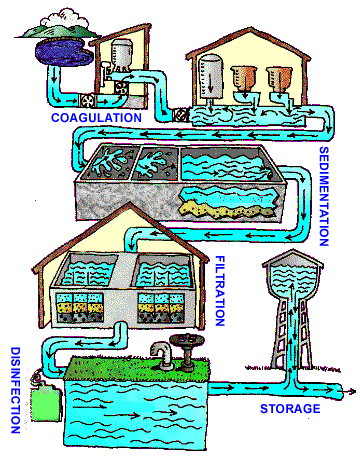
With few exceptions, all public water in the U.S. must go through this conventional drinking water process before it comes out of the tap. The first step, of course, is the smart selection of drinking water sources. The first step in the treatment process is called coagulation. During coagulation, chemicals are added to the water that make dirt and some dissolved particles stick together, resulting in clumps that are referred to as flocs. During the next process, sedimentation, the flocs start get really heavy and sink to the bottom of the tank where they can be removed. After coagulation and sedimentation comes filtration. During this step in the water treatment process, all of the water being treated flows slowly through filters made up of layers of sand, gravel and charcoal (Refer to the hands-on activity A Matter of Leaching to have students design and implement their own water filters). Following filtration is the final step in the treatment process: disinfection. During disinfection, chlorine is added. Chlorine is the same thing that is used to disinfect swimming pools, but when it is used for drinking water disinfection, much less is added. The law states that a certain amount of chlorine must be present in your water every time you turn your tap on. This requirement exists because some dangerous microorganisms that are able to get through all of the other treatment processes are killed by chlorine.
High DOM concentrations are very difficult to remove from water. Because DOM is dissolved in water, it requires a chemical reaction (or reactions) to remove it. Problems during the disinfection process can result if high amounts of DOM are in the water. They can form what are called disinfection byproducts (DBPs), which are harmful if ingested. One common DPB is chloroform (you may have seen a movie or television show in which a cloth is put over someone's mouth or nose and then the person passes out—which is due to breathing in chloroform!).
Associated Activities
- A Matter of Leaching - Students design and build simple water filters. Then, they test their filters using dirty water.
Lesson Closure
Water is an essential resource on earth. There are many different types of water sources and they all have different characteristics, which can affect their ability to become drinking water sources. One of the most important characteristics to consider when choosing a drinking water source is dissolved organic matter content. We learned that water with a high DOM content can have lots of other things in the water, such as microorganisms and metals, which would make the water much harder to treat! We also talked about the drinking water treatment process and learned about all the phases that your water goes through before it comes out of your tap. In order, those processes were: coagulation, sedimentation, filtration, disinfection, and storage. It's important to realize that if any of these steps were skipped, our water would not be clean enough to drink and could make people very sick.
Vocabulary/Definitions
aquifer: Underground storage unit for water.
coagulation: Process in drinking water treatment during which chemicals are added to the water that make dirt and particles stick together.
disinfection: Process in drinking water treatment during which chlorine is added to the water before it flows to taps.
disinfection byproduct: Formed when chlorine is added to water with high DOM.
dissolved organic matter (DOM): Result of decomposition of plants and animals in a body of water.
erosion: The process by which the surface of the earth is worn away by the action of water, glaciers, winds, waves, etc.
filtration: Process of running water through a filter to make it cleaner.
floc: When particles group together into one larger particle.
groundwater: Water that infiltrates through soil into the ground.
lake: Relatively still, large body of water that has land on all sides.
river: Flowing body of water that has a destination (another river, lake, ocean).
sedimentation: Process in drinking water treatment where the clumps of particles formed in coagulation settle to the bottom of the tank.
stream: Flowing water that has defined bed and banks.
surface water: Any water located on Earth's surface.
Assessment
Quiz: After the lesson, administer the All About Water Worksheet to gauge student comprehension of the material covered.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the various methods developed by environmental engineers for treating drinking water in the United States.

Students learn about several possible scenarios of contamination to drinking water, which comes from many different sources, including surface water and groundwater. They analyze the movement of sample contaminants through groundwater, in a similar way to how environmental engineers analyze the phys...

Students learn about water quality testing and basic water treatment processes and technology options. Biological, physical and chemical treatment processes are addressed, as well as physical and biological water quality testing, including testing for bacteria such as E. coli.
References
People Cause More Soil Erosion Than All Natural Processes. Posted November 4, 2004. ScienceDaily, University of Michigan. Retrieved March 5, 2014 from www.sciencedaily.com/releases/2004/11/041103234736.htm
Copyright
© 2012 by Regents of the University of ColoradoContributors
Jessica Ebert; Marissa H. ForbesSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: February 25, 2020




User Comments & Tips